
Choose the statements that are true about polymers from the given options.
A. Teflon is prepared by heating tetrafluoroethene in the presence of a persulfate catalyst at high pressure.
B. Natural rubber is polyisoprene containing trans-alkene units.
C. Nylon-6 has amide linkages
D. Cellulose has only $\alpha $- D – glucose units that are joined by glycosidic linkages
Answer
578.4k+ views
Hint: Think about each of the options given separately as they are not related to each other than the fact that they are polymers. Consider the structures and preparations of all the polymers given and then answer the question.
Complete step by step solution:
We will look at the structures of the molecules mentioned in the options one by one and determine whether the given statements about them are true or not.
- Option A
To answer this question, we will have to look at how Teflon is prepared, what the monomer is, and what catalysts are used. Teflon is also known as polytetrafluoroethylene and it is prepared using the tetrafluoroethene molecules. These molecules need to be heated at high temperature and pressure in the presence of a persulfate catalyst like benzoyl peroxide to actually form the teflon polymer. The reaction for the formation of Teflon is as follows:

Thus, option A is true.
- Option B
To determine whether option B is true or not, we need to look at the structure of natural rubber. The structure is:
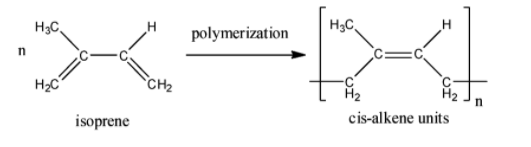
Here, we can see that natural rubber has cis alkene units due to the fact that it is formed from cis-isoprene molecules. Isoprene is the common name for the molecule 2-methylbuta-1,3-diene. If natural rubber had trans-alkene units, it would look something like this:
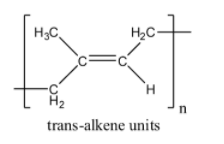
Thus, we can say that option B is false since rubber has cis units and not trans units.
- Option C
To solve this, let us directly look at the structure of Nylon-6 since the method of preparation will not determine the presence of linkages. The structure of Nylon-6 is as follows:
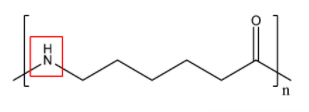
Here, we can see the presence of an amide group that is involved in the linkage of two monomers that form the polymer. Thus, we can say that nylon-6 has amide linkages and option C is true.
- Option D
To check whether the statement given in option D is true or not, we will have to look at the monomeric unit of cellulose so that we can confirm the presence of the glycosidic linkages and the $\alpha $- D – glucose molecules. The structure of cellulose is:

Here, note that the glycosidic linkage is present between the two molecules of glucose but now we have to determine what type of glucose molecule is present. Note that the cellulose is formed by the dehydration of glucose, so one water molecule has already been removed. The hydrogen from the hydroxyl group from the ring on the left is removed and the hydroxyl group from the ring on the right is removed to form the glycosidic linkage. Note that the hydroxyl group is present above the plane of the ring on the anomeric carbon; this indicates that the given molecule is a $\beta $- D – glucose molecule. Thus, option D is not true.
Hence, in this question, option A and option C are true.
Note: Note that the anomeric carbon is the carbon labeled as 1 and is found directly next to the oxygen atom in the pyranose ring. If the hydroxyl group on that carbon is present above the plane of the ring, then we call the molecule the $\beta $- anomer of D-glucose and if the hydroxyl group is present below the plane of the ring then it is called the $\alpha $- anomer of D-glucose.
Complete step by step solution:
We will look at the structures of the molecules mentioned in the options one by one and determine whether the given statements about them are true or not.
- Option A
To answer this question, we will have to look at how Teflon is prepared, what the monomer is, and what catalysts are used. Teflon is also known as polytetrafluoroethylene and it is prepared using the tetrafluoroethene molecules. These molecules need to be heated at high temperature and pressure in the presence of a persulfate catalyst like benzoyl peroxide to actually form the teflon polymer. The reaction for the formation of Teflon is as follows:

Thus, option A is true.
- Option B
To determine whether option B is true or not, we need to look at the structure of natural rubber. The structure is:

Here, we can see that natural rubber has cis alkene units due to the fact that it is formed from cis-isoprene molecules. Isoprene is the common name for the molecule 2-methylbuta-1,3-diene. If natural rubber had trans-alkene units, it would look something like this:

Thus, we can say that option B is false since rubber has cis units and not trans units.
- Option C
To solve this, let us directly look at the structure of Nylon-6 since the method of preparation will not determine the presence of linkages. The structure of Nylon-6 is as follows:

Here, we can see the presence of an amide group that is involved in the linkage of two monomers that form the polymer. Thus, we can say that nylon-6 has amide linkages and option C is true.
- Option D
To check whether the statement given in option D is true or not, we will have to look at the monomeric unit of cellulose so that we can confirm the presence of the glycosidic linkages and the $\alpha $- D – glucose molecules. The structure of cellulose is:

Here, note that the glycosidic linkage is present between the two molecules of glucose but now we have to determine what type of glucose molecule is present. Note that the cellulose is formed by the dehydration of glucose, so one water molecule has already been removed. The hydrogen from the hydroxyl group from the ring on the left is removed and the hydroxyl group from the ring on the right is removed to form the glycosidic linkage. Note that the hydroxyl group is present above the plane of the ring on the anomeric carbon; this indicates that the given molecule is a $\beta $- D – glucose molecule. Thus, option D is not true.
Hence, in this question, option A and option C are true.
Note: Note that the anomeric carbon is the carbon labeled as 1 and is found directly next to the oxygen atom in the pyranose ring. If the hydroxyl group on that carbon is present above the plane of the ring, then we call the molecule the $\beta $- anomer of D-glucose and if the hydroxyl group is present below the plane of the ring then it is called the $\alpha $- anomer of D-glucose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




