
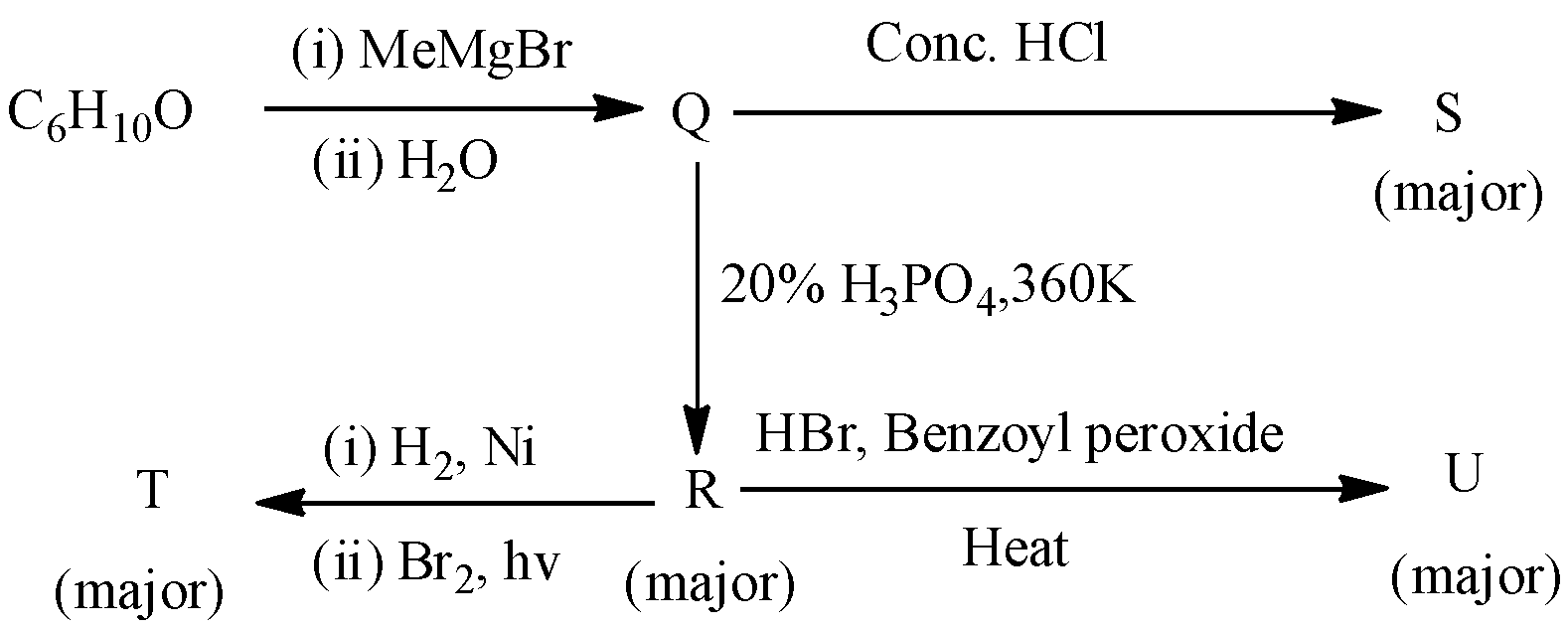
Choose the correct option(s) for the following set of reactions
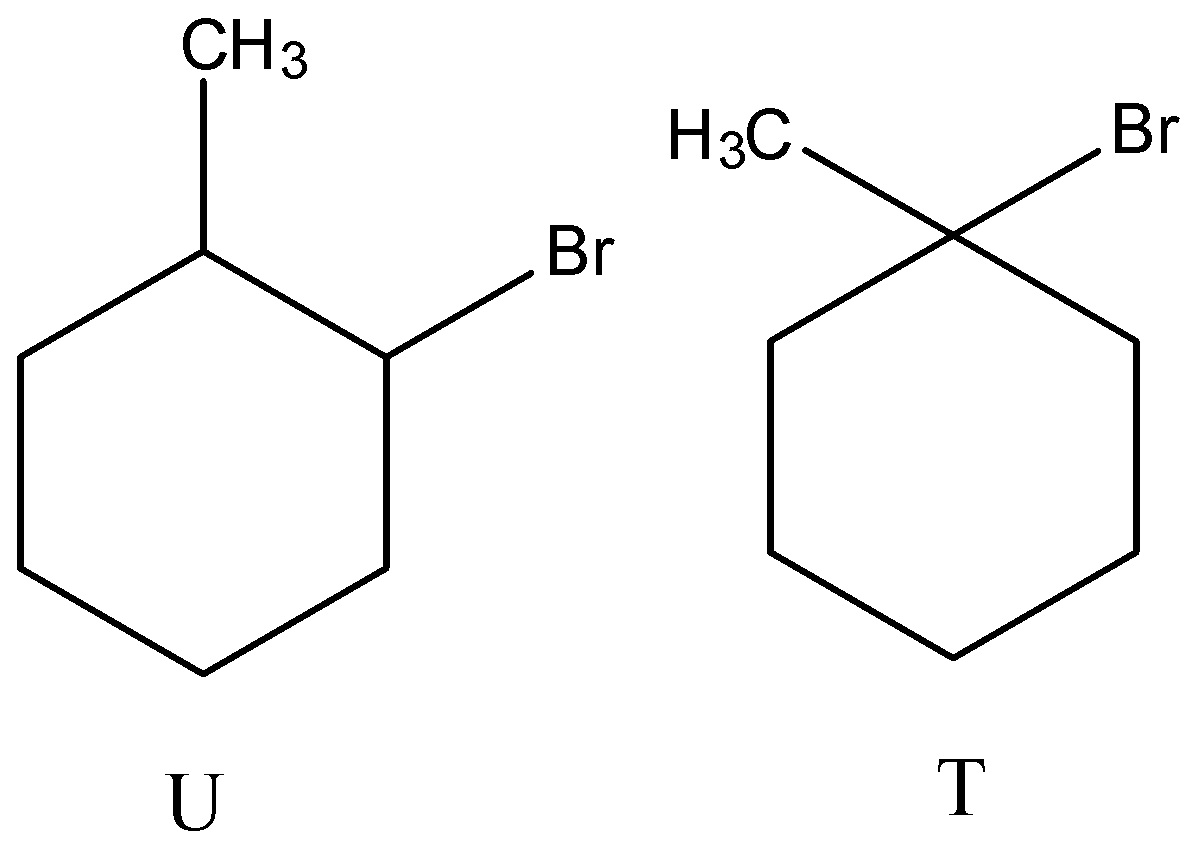
(A)
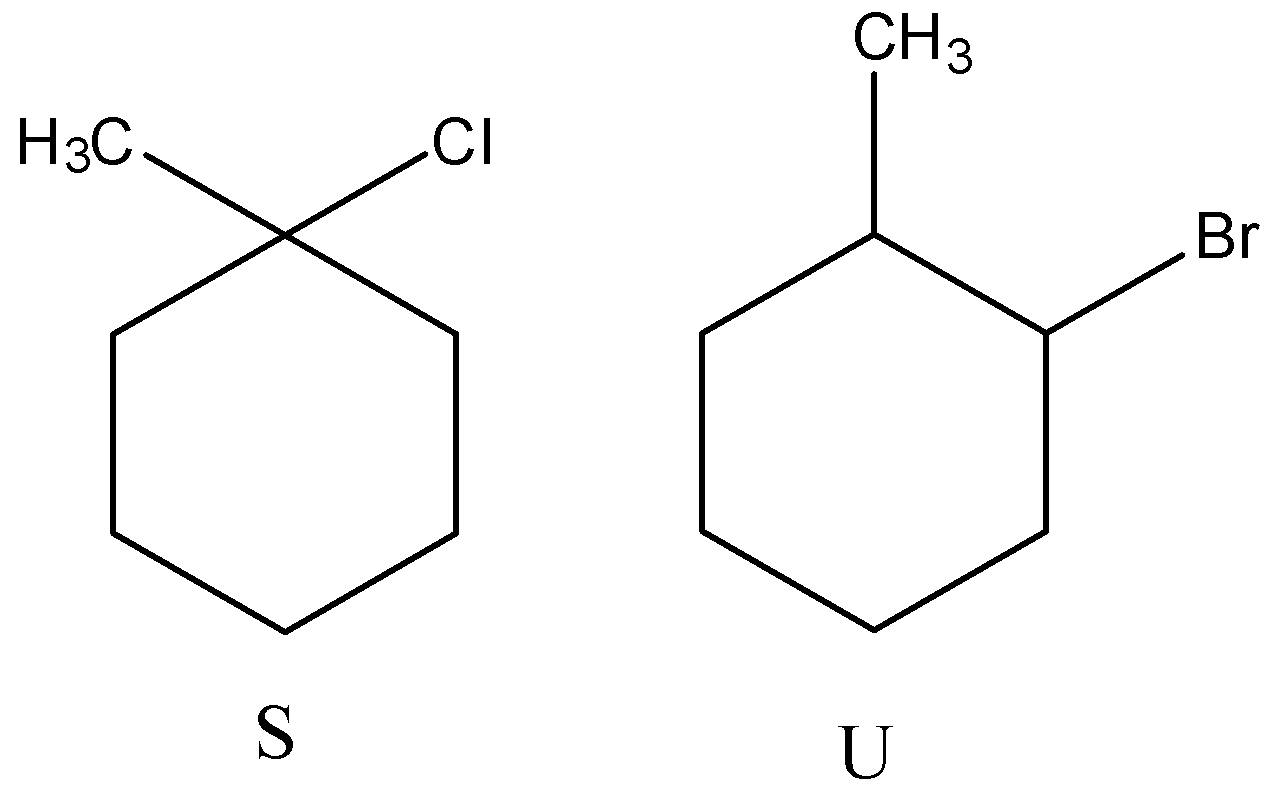
(B)
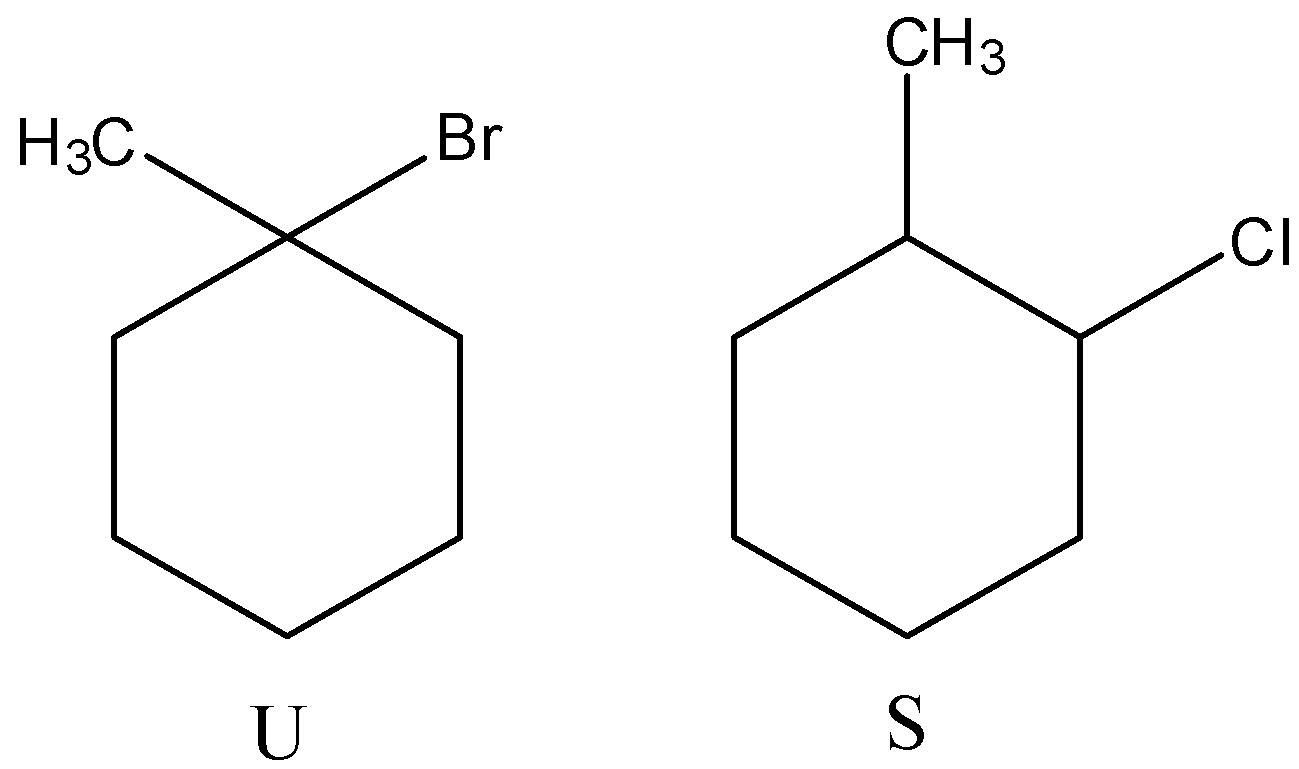
(C)
(D)





Answer
585k+ views
Hint: MeMgBr is called a Grignard reagent. Grignard reagent reacts with carbonyl compounds and forms alcohols as the product. Nickel is a catalyst, in presence of hydrogen gas nickel converts unsaturated hydrocarbons to saturated hydrocarbons.
Complete step by step answer:
- In the Given question there is an involvement of several organic reactions.
- We will start discussing from the starting reaction onwards.
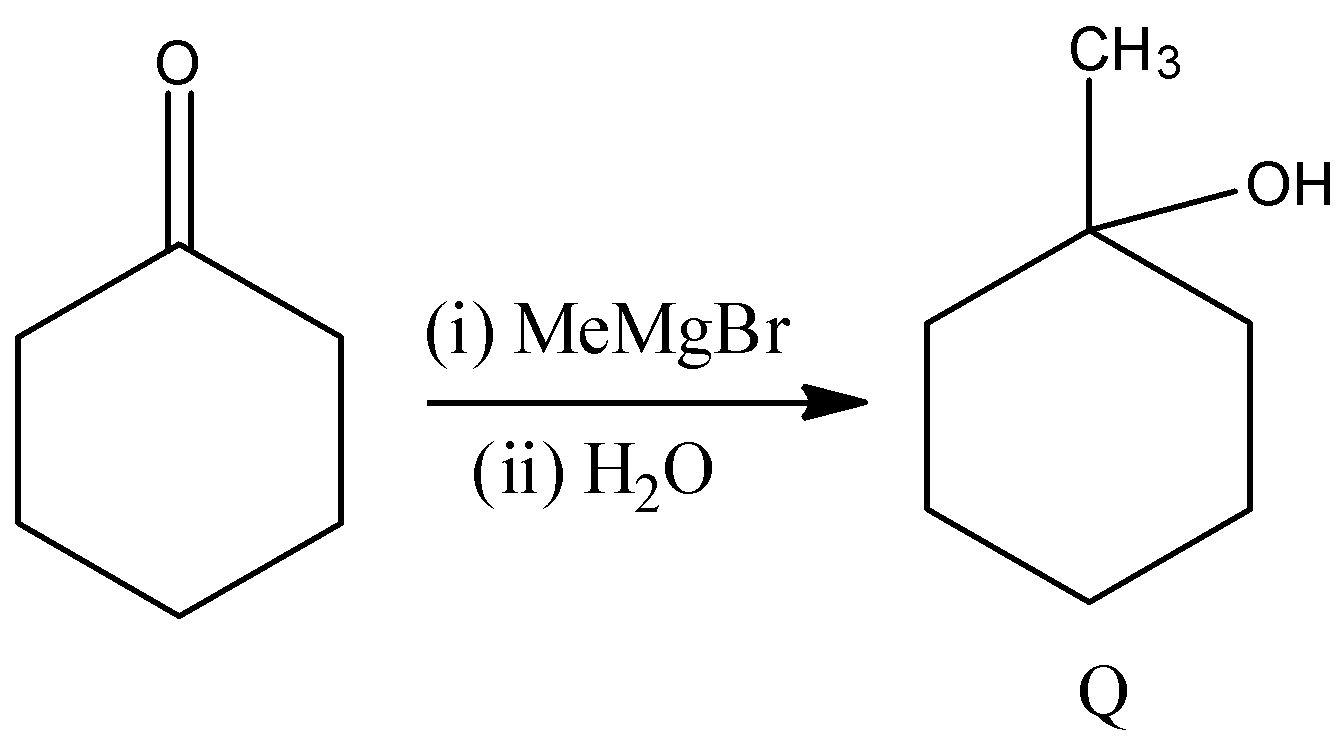
- In the above reaction Grignard reagent reacts with cyclohexanone and forms corresponding alcohol as the product.
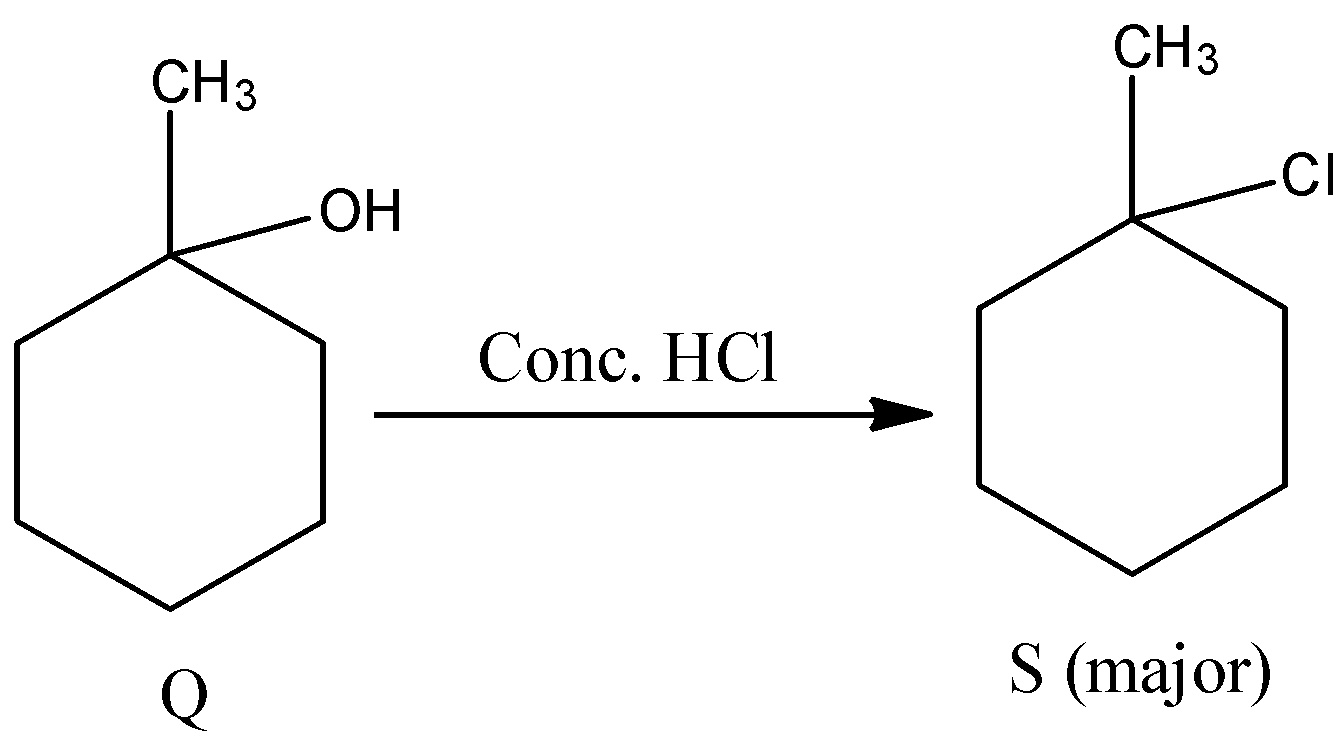
- The product formed in the above reaction (Q) reacts with Conc. HCl forms a chloro derivative as the product. The chemical reaction is as follows.
- The chemical (Q) reacts with 20% phosphoric acid and forms the R. The chemical reaction of Q with 20% phosphoric acid at 360 K is as follows.
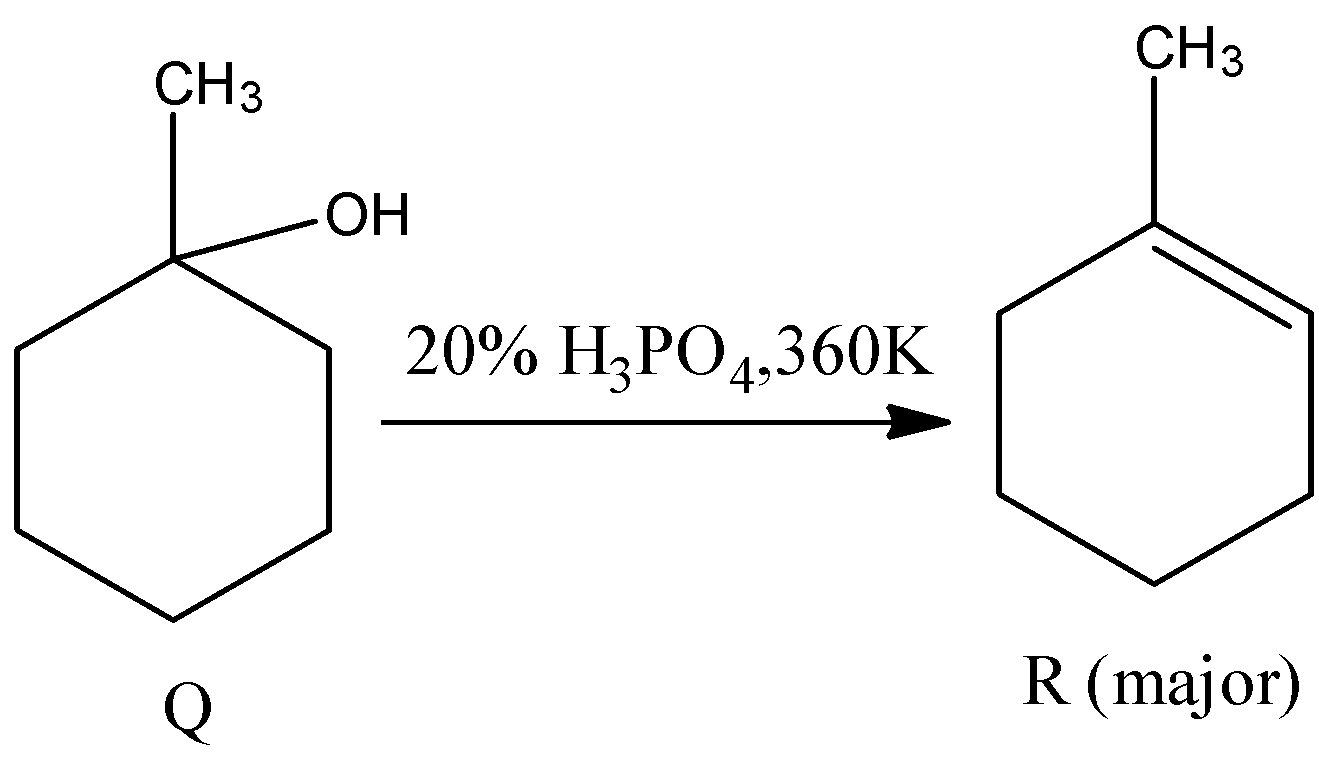
- In the above reaction phosphoric acid acts as a dehydrating agent and removes water from Q to get R.
- The compound R reacts with hydrogen gas in presence of nickel catalyst and bromine gas in presence of sunlight gives a bromo derivative as the product.
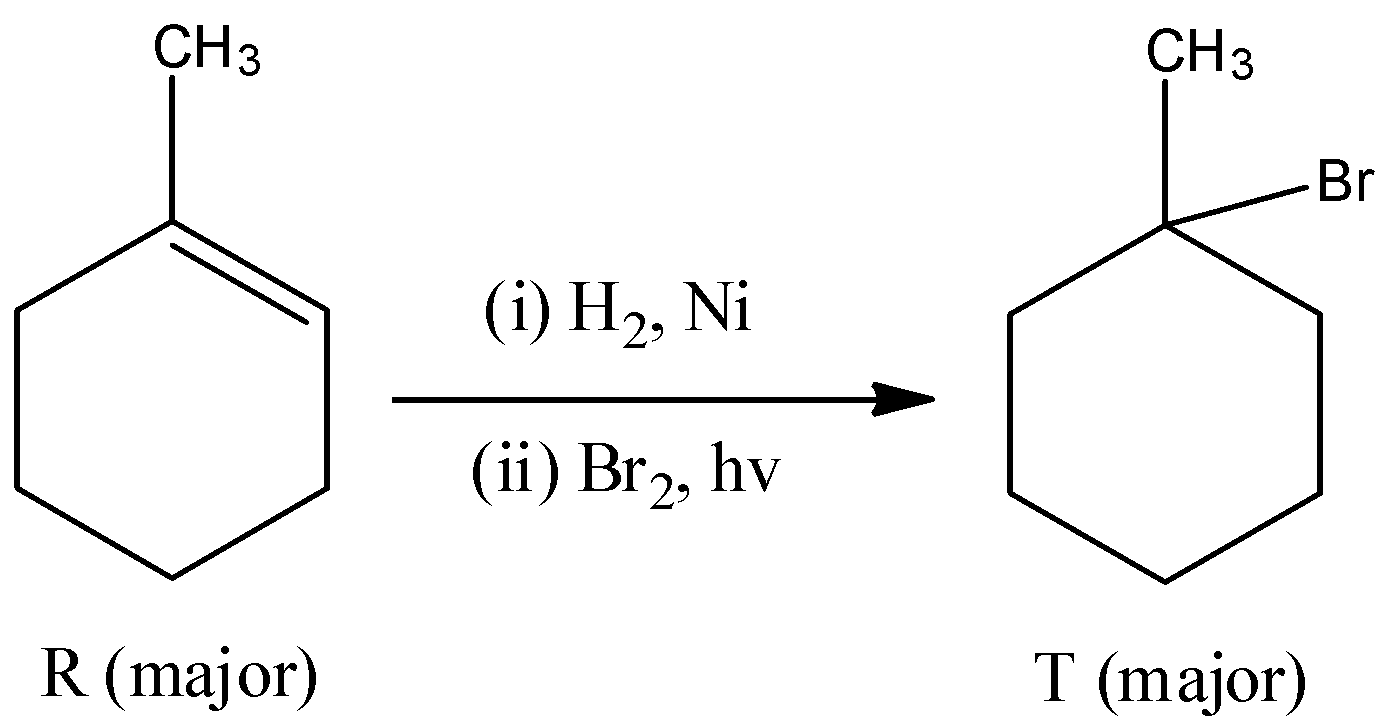
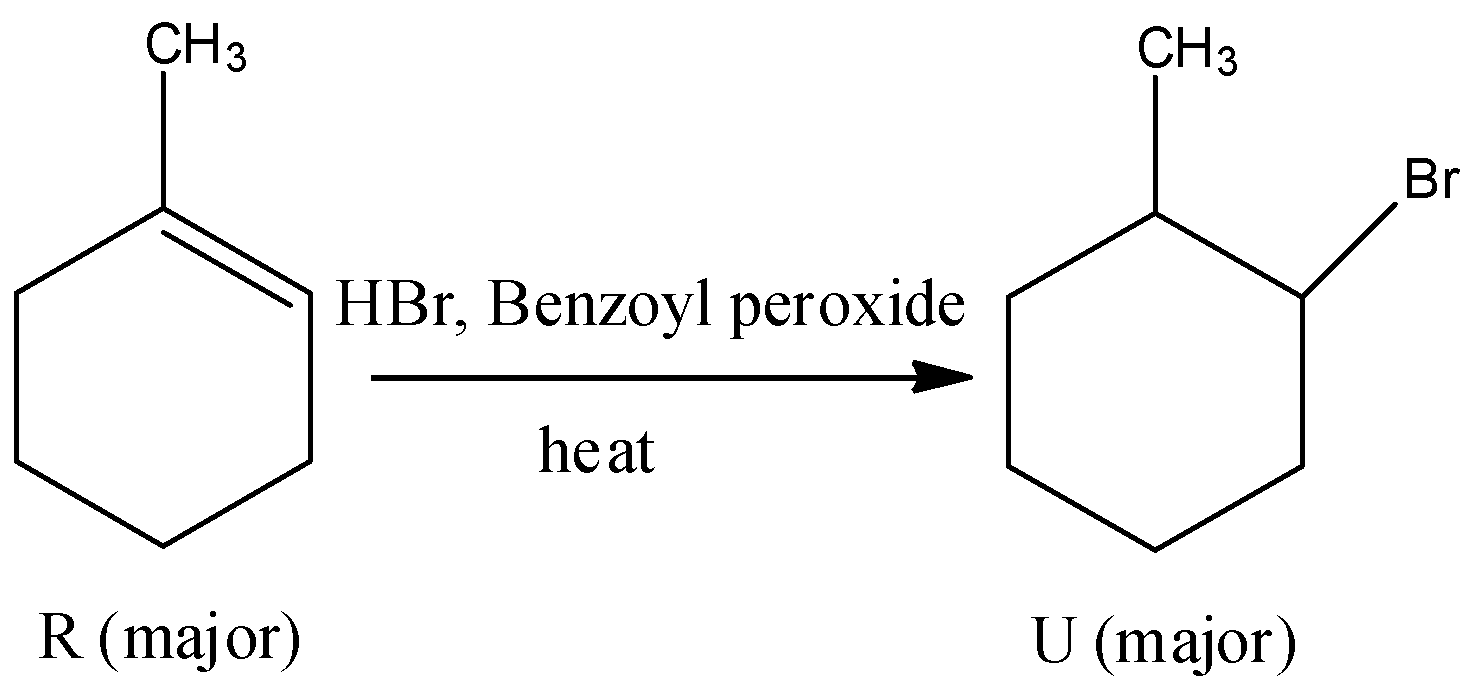
- The product R reacts with hydrogen bromide in presence of benzoyl peroxide to give a bromo derivative. The compound R undergoes free radical addition reaction in presence of benzoyl peroxide. The chemical reaction is as follows.
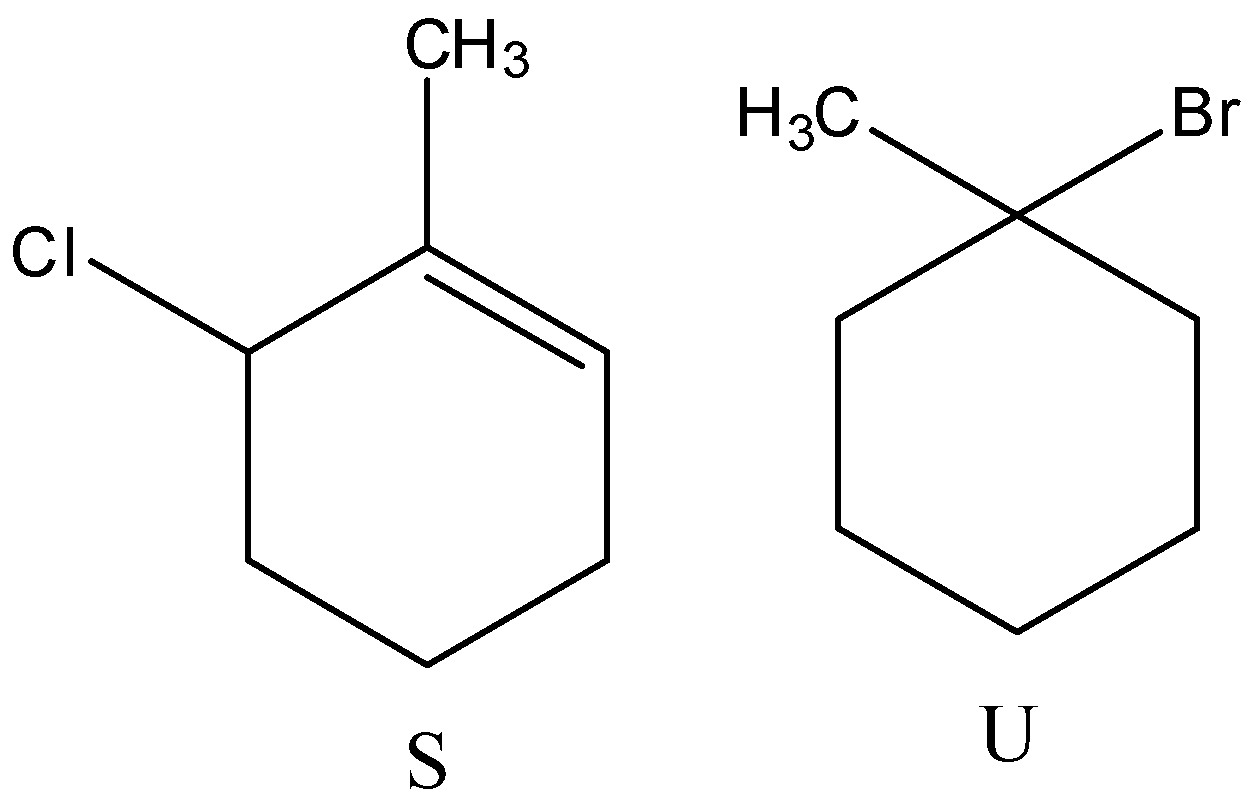
- Therefore by observing the above reactions we can say that the products are as follows.
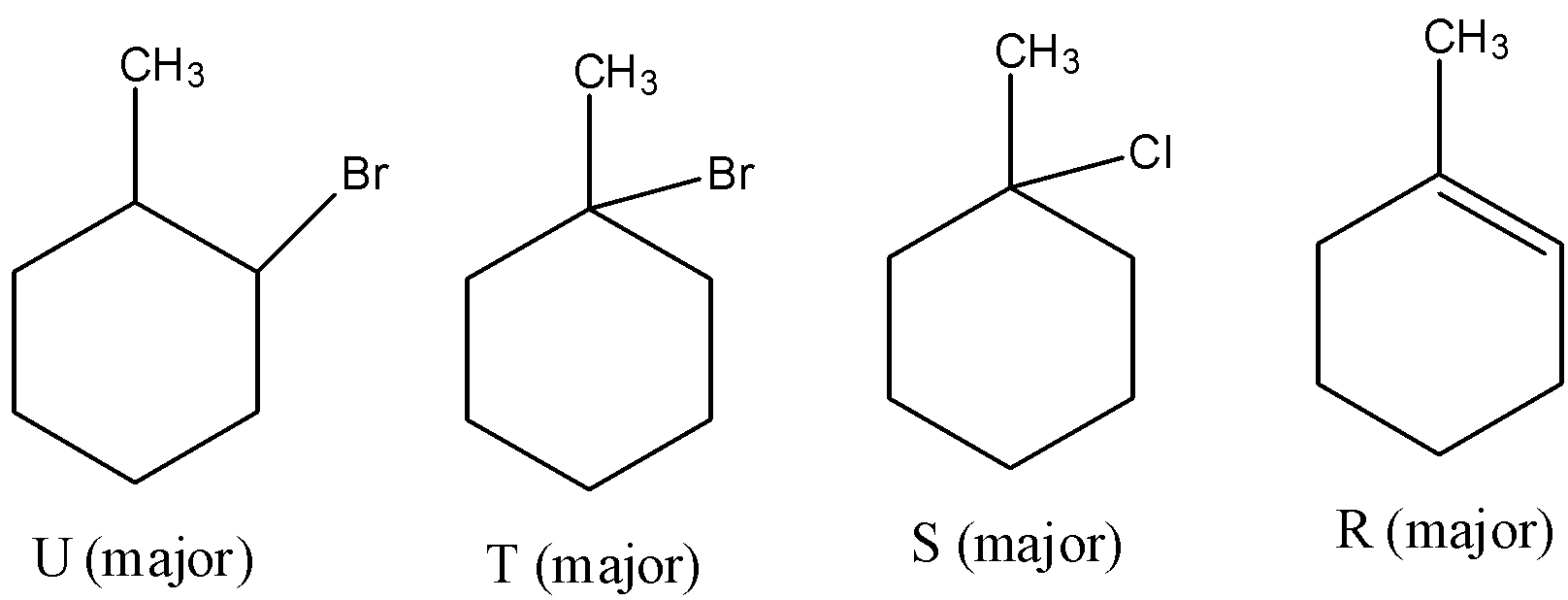
- So, the correct options are both C and D.
Note: We can see that the product R forms two different bromo derivatives called T and U. The precursor for compounds T and U is the same. But the products are different (T and U) because of the usage of reagent during the reaction.
Complete step by step answer:
- In the Given question there is an involvement of several organic reactions.
- We will start discussing from the starting reaction onwards.

- In the above reaction Grignard reagent reacts with cyclohexanone and forms corresponding alcohol as the product.
- The product formed in the above reaction (Q) reacts with Conc. HCl forms a chloro derivative as the product. The chemical reaction is as follows.

- The chemical (Q) reacts with 20% phosphoric acid and forms the R. The chemical reaction of Q with 20% phosphoric acid at 360 K is as follows.

- In the above reaction phosphoric acid acts as a dehydrating agent and removes water from Q to get R.
- The compound R reacts with hydrogen gas in presence of nickel catalyst and bromine gas in presence of sunlight gives a bromo derivative as the product.

- The product R reacts with hydrogen bromide in presence of benzoyl peroxide to give a bromo derivative. The compound R undergoes free radical addition reaction in presence of benzoyl peroxide. The chemical reaction is as follows.

- Therefore by observing the above reactions we can say that the products are as follows.

- So, the correct options are both C and D.
Note: We can see that the product R forms two different bromo derivatives called T and U. The precursor for compounds T and U is the same. But the products are different (T and U) because of the usage of reagent during the reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




