
Choose the correct option from the following:
A. In the electrophilic substitution of toluene with $B{{r}_{2}}$, iron (III) bromide acts as a Lewis acid
B. In the reaction of toluene with $C{{l}_{2}}/FeC{{l}_{3}}$, ortho and para isomers are easily separated
C. Similar reaction with iodine is reversible in nature.
D. All of these
Answer
594.9k+ views
Hint: Think about what Lewis acids are and how they function in a reaction. Consider the physical properties of ortho and para isomers like boiling point and melting point. What the bulky nature of iodine will cause in reactions
Complete answer:
First, we will see how bromine interacts with $FeB{{r}_{3}}$.
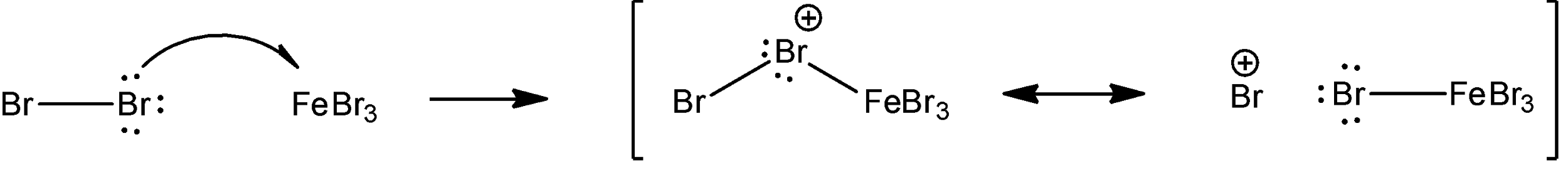
Here, we can see that $FeB{{r}_{3}}$ accepts an electron pair from bromine. Molecules are classified as Lewis acids if they can accept a pair of non-bonding electrons i.e. a lone pair.
Hence, ‘A. In the electrophilic substitution of toluene with $B{{r}_{2}}$, iron (III) bromide acts as a Lewis acid’ is true.
Now, we will see how the bromine ion attacks toluene at the ortho position and its resonating structures.
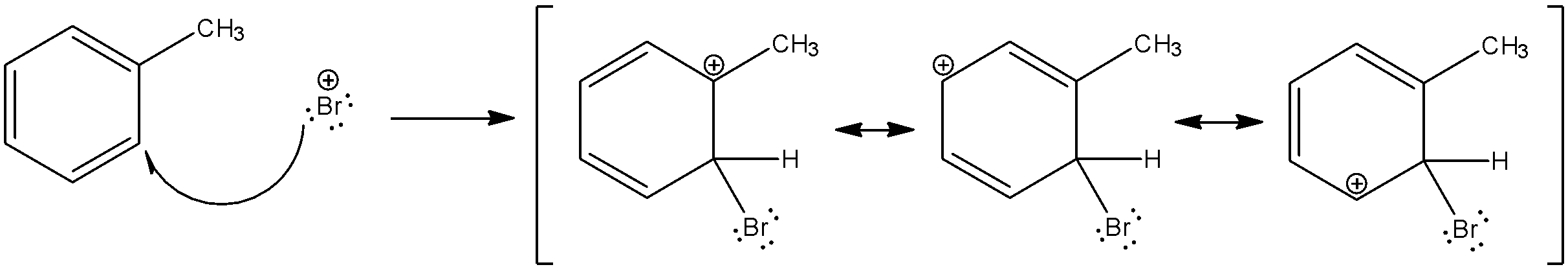
The bromine ion can attack toluene at the meta and para positions too and form similar resonance structures. But, the resonance structures of the ortho and para structures are much more stable than the meta structure. Thus, both these isomers are preferred. There is a vast difference in the boiling points of the ortho and para isomers of all halo toluenes and can be easily separated by physical methods.
Hence, ‘B. In the reaction of toluene with $C{{l}_{2}}/FeC{{l}_{3}}$, ortho and para isomers are easily separated’ is true.
Now, one of the resonating structures will interact with the iron-bromide complex and form the final product along with hydrobromic acid.
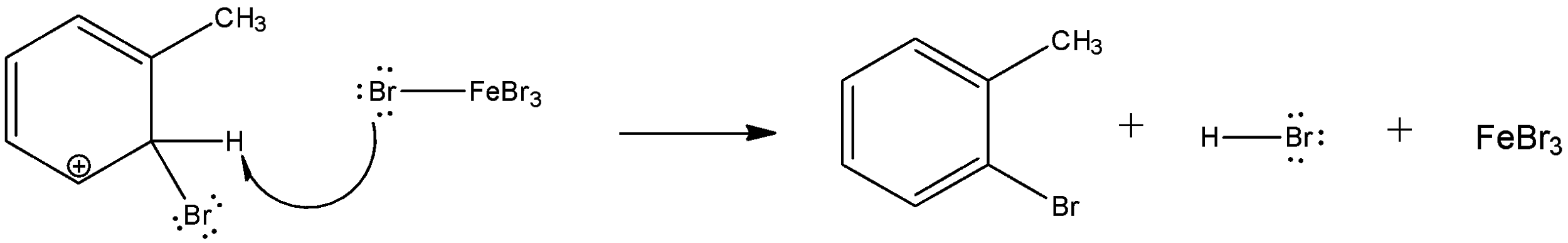
Reaction with chlorine will form hydrochloric acid and reaction with iodine will form hydroiodic acid. But, since iodine is a bulky atom, its reaction with toluene is easily reversible since it is less stable.
Hence, ‘C. Similar reaction with iodine is reversible in nature.’ is true.
The net general, unbalanced reaction of toluene with bromine is as follows:
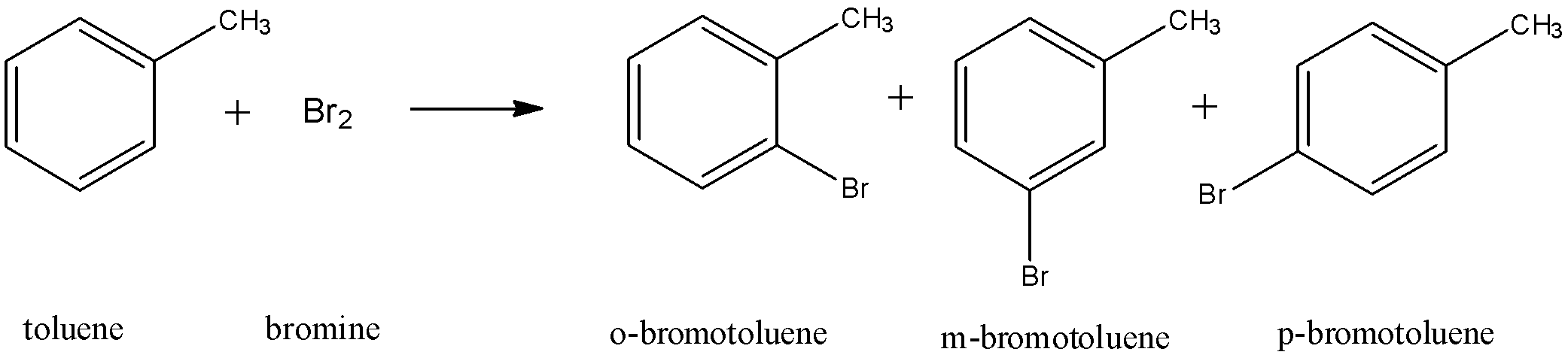
Hence, the correct answer is ‘D. All of these’ since all the previous options are true.
Note: The formation of the para isomer is the most in this reaction. The formation of the meta isomer is the least due to unstable resonance structures. The stability of the ortho isomers is slightly less than the para isomers due to steric hindrance. But it is possible to separate all these isomers by purely physical methods since their physical properties show a noticeable difference.
Complete answer:
First, we will see how bromine interacts with $FeB{{r}_{3}}$.

Here, we can see that $FeB{{r}_{3}}$ accepts an electron pair from bromine. Molecules are classified as Lewis acids if they can accept a pair of non-bonding electrons i.e. a lone pair.
Hence, ‘A. In the electrophilic substitution of toluene with $B{{r}_{2}}$, iron (III) bromide acts as a Lewis acid’ is true.
Now, we will see how the bromine ion attacks toluene at the ortho position and its resonating structures.

The bromine ion can attack toluene at the meta and para positions too and form similar resonance structures. But, the resonance structures of the ortho and para structures are much more stable than the meta structure. Thus, both these isomers are preferred. There is a vast difference in the boiling points of the ortho and para isomers of all halo toluenes and can be easily separated by physical methods.
Hence, ‘B. In the reaction of toluene with $C{{l}_{2}}/FeC{{l}_{3}}$, ortho and para isomers are easily separated’ is true.
Now, one of the resonating structures will interact with the iron-bromide complex and form the final product along with hydrobromic acid.

Reaction with chlorine will form hydrochloric acid and reaction with iodine will form hydroiodic acid. But, since iodine is a bulky atom, its reaction with toluene is easily reversible since it is less stable.
Hence, ‘C. Similar reaction with iodine is reversible in nature.’ is true.
The net general, unbalanced reaction of toluene with bromine is as follows:

Hence, the correct answer is ‘D. All of these’ since all the previous options are true.
Note: The formation of the para isomer is the most in this reaction. The formation of the meta isomer is the least due to unstable resonance structures. The stability of the ortho isomers is slightly less than the para isomers due to steric hindrance. But it is possible to separate all these isomers by purely physical methods since their physical properties show a noticeable difference.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




