
Chlorobenzene can be converted to benzene by treatment with $NaOH/CaO$.
(a) True
(b) False
Answer
575.1k+ views
Hint: When chlorobenzene is made to react with the sodium hydroxide or the calcium oxide, it results in the formation of the compound which is aromatic in nature and consists of oxygen and sodium atoms attached to it. Now with the help of this, you can easily identify whether the given statement is correct or wrong.
Complete answer:
First of all, let’s discuss chlorobenzene. Chlorobenzene is an aromatic compound. When chlorine is attached to the benzene ring, then the aromatic compound is known as chlorobenzene.
Now considering the statement;
When chlorobenzene is made to undergo reaction with the sodium hydroxide i.e. NaOH , it results in the formation of the sodium phenoxide. The reaction is supposed to occur as;
${{C}_{6}}{{H}_{5}}Cl+NaOH\to {{C}_{6}}{{H}_{5}}ONa$
So, thus Chlorobenzene cannot be converted to benzene by treatment with $NaOH/CaO$ and the given statement is false.
Hence, option (b) is correct.
Additional information:
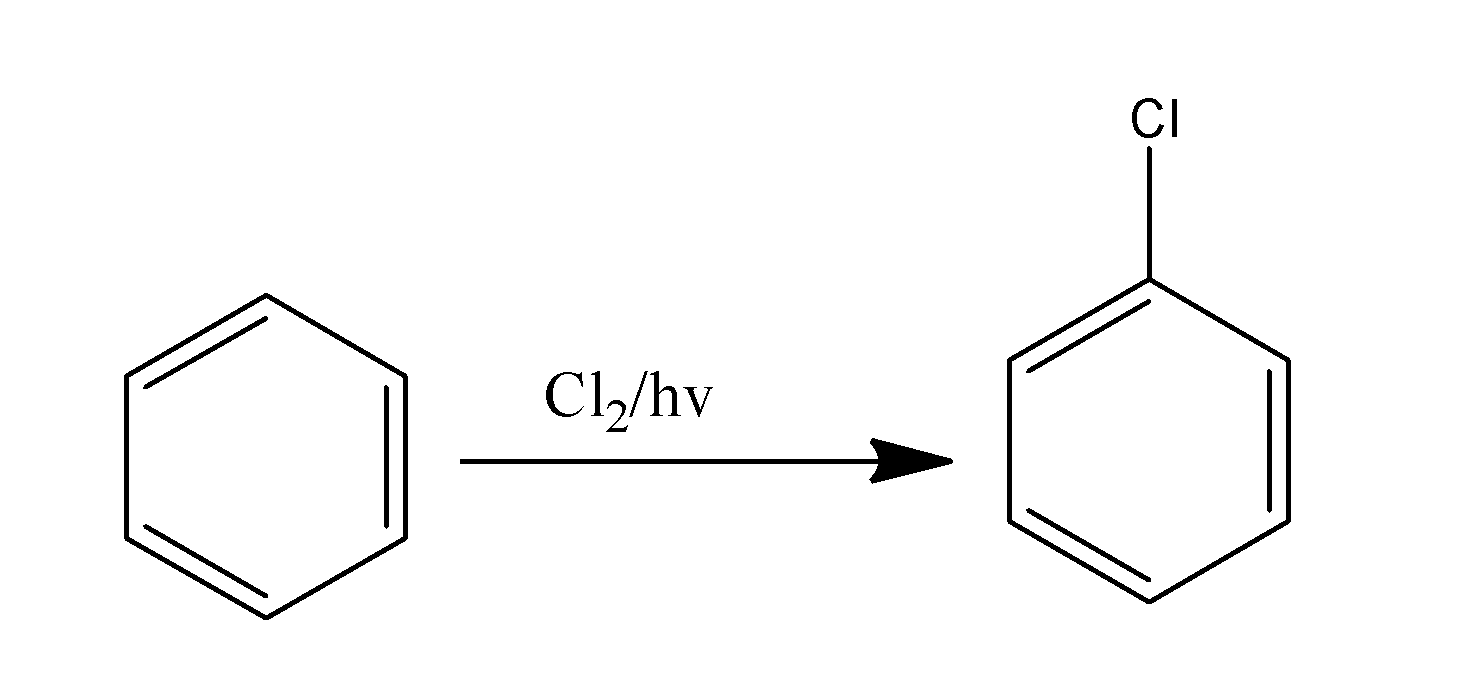
Chlorobenzene, itself, is formed by the halogenation of the benzene ring in the presence of light. The benzene ring undergoes electrophilic substitution reaction and the hydrogen atom of the benzene ring is replaced by the chlorine atom, thus, resulting in the formation of the chlorobenzene. The reaction is supposed to occur as-
Note:
Chlorobenzene when is treated with the hydrogen in the presence of nickel alloy and sodium hydroxide, it results in the formation of the benzene ring. The reaction is supposed to occur as;
$\,{{C}_{6}}{{H}_{5}}Cl+2H\xrightarrow[NaOH]{Ni-alloy}{{C}_{6}}{{H}_{6}}+HCl$
Complete answer:
First of all, let’s discuss chlorobenzene. Chlorobenzene is an aromatic compound. When chlorine is attached to the benzene ring, then the aromatic compound is known as chlorobenzene.
Now considering the statement;
When chlorobenzene is made to undergo reaction with the sodium hydroxide i.e. NaOH , it results in the formation of the sodium phenoxide. The reaction is supposed to occur as;
${{C}_{6}}{{H}_{5}}Cl+NaOH\to {{C}_{6}}{{H}_{5}}ONa$
So, thus Chlorobenzene cannot be converted to benzene by treatment with $NaOH/CaO$ and the given statement is false.
Hence, option (b) is correct.
Additional information:
Chlorobenzene, itself, is formed by the halogenation of the benzene ring in the presence of light. The benzene ring undergoes electrophilic substitution reaction and the hydrogen atom of the benzene ring is replaced by the chlorine atom, thus, resulting in the formation of the chlorobenzene. The reaction is supposed to occur as-

Note:
Chlorobenzene when is treated with the hydrogen in the presence of nickel alloy and sodium hydroxide, it results in the formation of the benzene ring. The reaction is supposed to occur as;
$\,{{C}_{6}}{{H}_{5}}Cl+2H\xrightarrow[NaOH]{Ni-alloy}{{C}_{6}}{{H}_{6}}+HCl$
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

The largest wind power cluster is located in the state class 11 social science CBSE

Explain zero factorial class 11 maths CBSE

State and prove Bernoullis theorem class 11 physics CBSE

What steps did the French revolutionaries take to create class 11 social science CBSE

Which among the following are examples of coming together class 11 social science CBSE




