
Chlorine on reaction with hot and concentrated sodium hydroxide gives:
A. $C{{l}^{-}}\text{ and Cl}{{\text{O}}_{2}}^{-}$
B. $C{{l}^{-}}\text{ and Cl}{{\text{O}}_{3}}^{-}$
C. $C{{l}^{-}}\text{ and Cl}{{\text{O}}^{-}}$
D. $Cl{{O}_{3}}^{-}\text{ and Cl}{{\text{O}}_{2}}^{-}$
Answer
565.5k+ views
Hint: Here in the question, chlorine gas is reacting with hot and concentrated NaOH acid. So, there will not be only 1 product. Several products will be formed in this reaction as the concentration of the reactants is very much.
Complete step by step solution:
- The reaction of the same 2 compounds can result in different products depending on several factors like the concentration of the reactants, temperature of the reaction, pressure of the reaction and certain catalysts.
-Order of a reaction is responsible for the type of reaction that occurs between the different products. It decides the mechanism of the reaction and so different products are obtained through the reactants are the same.
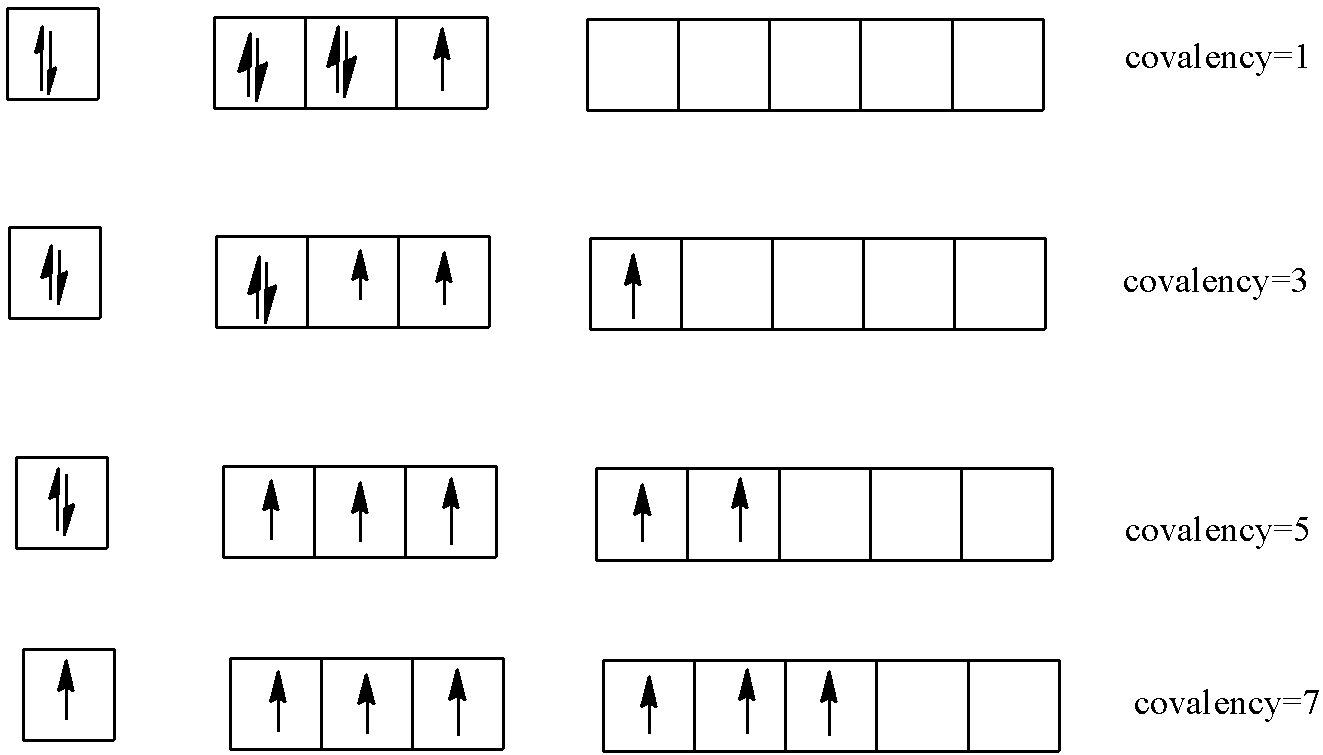
-Same thing occurs in this reaction. Chlorine gas different products with cold NaOH, hot and dilute NaOH and hot and concentrated NaOH. This is possible in chlorine as it has vacant d-orbitals and so it can have variable covalencies. There are 7 electrons in normal state in a chlorine molecule. In different excitation states, its covalencies change.
-When cold and diluted NaOH is used, then the chlorine molecule is not able to oxidize completely but when hot and concentrated NaOH is used, chlorine is able to oxidize completely to its maximum covalency possible.
-One chlorine atom oxidizes and the other atom reduces and so different products are formed which have different oxidation states. So here we see that the same atom is oxidized as well as reduced and so it is called auto or self redox reaction.
-Thus we see that chlorine can have different oxidation states and this makes it capable to form different compounds with different products. The reaction can be shown as
$6NaOH+3C{{l}_{2}}\to 5NaCl+NaCl{{O}_{3}}+3{{H}_{2}}O$
-Here we can see that the oxidation state of chlorine gas is 0, chlorine in NaCl is -1 and in $NaCl{{O}_{3}}$ is +5. So, chlorine to $NaCl{{O}_{3}}$ is oxidation reaction and chlorine to NaCl is reduction reaction. Both oxidation and reduction are occurring in the same reaction and so the reaction is a redox reaction. Chlorine is showing disproportionation.
Therefore the correct option is B.
Note: If the reaction of chlorine occurs with cold and dilute NaOH then the products formed will be different. Chlorine will not be able to oxidize completely. The reaction for it can be shown as
$\begin{align}
& 2NaOH+C{{l}_{2}}\to NaCl+NaCl{{O}_{{}}}+{{H}_{2}}O \\
& \\
\end{align}$
Complete step by step solution:
- The reaction of the same 2 compounds can result in different products depending on several factors like the concentration of the reactants, temperature of the reaction, pressure of the reaction and certain catalysts.
-Order of a reaction is responsible for the type of reaction that occurs between the different products. It decides the mechanism of the reaction and so different products are obtained through the reactants are the same.
-Same thing occurs in this reaction. Chlorine gas different products with cold NaOH, hot and dilute NaOH and hot and concentrated NaOH. This is possible in chlorine as it has vacant d-orbitals and so it can have variable covalencies. There are 7 electrons in normal state in a chlorine molecule. In different excitation states, its covalencies change.
-When cold and diluted NaOH is used, then the chlorine molecule is not able to oxidize completely but when hot and concentrated NaOH is used, chlorine is able to oxidize completely to its maximum covalency possible.
-One chlorine atom oxidizes and the other atom reduces and so different products are formed which have different oxidation states. So here we see that the same atom is oxidized as well as reduced and so it is called auto or self redox reaction.

-Thus we see that chlorine can have different oxidation states and this makes it capable to form different compounds with different products. The reaction can be shown as
$6NaOH+3C{{l}_{2}}\to 5NaCl+NaCl{{O}_{3}}+3{{H}_{2}}O$
-Here we can see that the oxidation state of chlorine gas is 0, chlorine in NaCl is -1 and in $NaCl{{O}_{3}}$ is +5. So, chlorine to $NaCl{{O}_{3}}$ is oxidation reaction and chlorine to NaCl is reduction reaction. Both oxidation and reduction are occurring in the same reaction and so the reaction is a redox reaction. Chlorine is showing disproportionation.
Therefore the correct option is B.
Note: If the reaction of chlorine occurs with cold and dilute NaOH then the products formed will be different. Chlorine will not be able to oxidize completely. The reaction for it can be shown as
$\begin{align}
& 2NaOH+C{{l}_{2}}\to NaCl+NaCl{{O}_{{}}}+{{H}_{2}}O \\
& \\
\end{align}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




