
Chloretone is formed when chloroform reacts with:
A) formaldehyde
B) acetaldehyde
C) acetone
D) benzaldehyde
Answer
535.5k+ views
Hint: Chloretone, having a chemical formula of \[{C_4}{H_7}C{l_3}O\], is actually chlorobutanol or chlorbutol. It appears as a white, volatile solid possessing a menthol like odour. It is basically an alcohol-based preservative having no surfactant activity. It also possesses sedative-hypnotic or weak local anesthetic actions apart from the antifungal and antibacterial properties.
Complete step by step solution:
Chloretone or chlorobutanol was synthesized for the first time in 1881 by a German chemist namely Conrad Willgerodt. The IUPAC name of chloretone is 1,1,1-Trichloro-2-methyl propan-2-ol. The reaction of acetone with chloroform in the presence of sodium or potassium hydroxide leads to the formation of chloretone. Actually, the anions present in the reactants i.e. \[C{H_3}C{O^ - }\] and \[CC{l_3}^ - \] reacts and form bonds with the cations i.e. \[{H^ + }\] and \[C{H_3}^ + \] respectively, which as a result, leads to the formation of chloretone. It may also undergo sublimation or recrystallization for further purification. The chemical equation for the formation of chloretone has been depicted below:
$C{H_3}COC{H_3} + CC{l_3}\xrightarrow{{KOH}}{C_4}{H_7}C{l_3}O$
It can be written as:
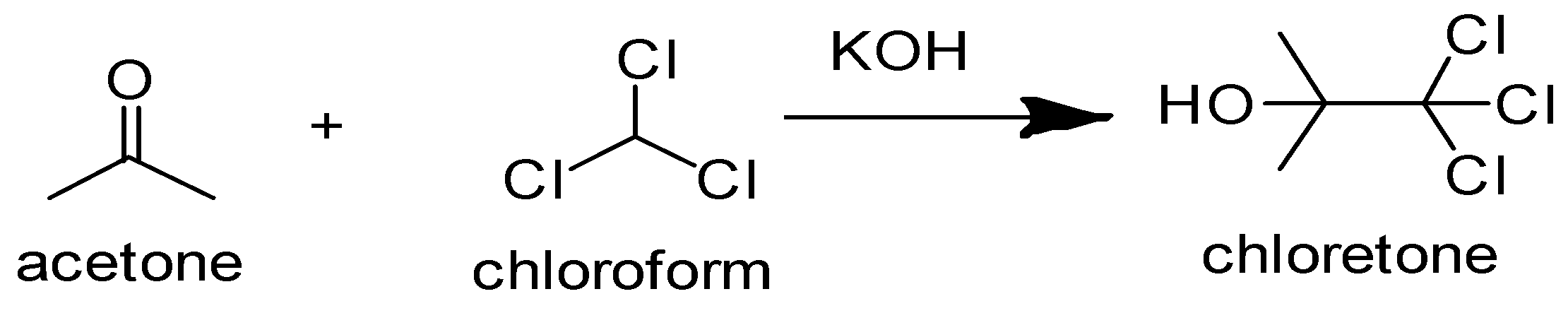
As a result, Chloretone is formed when chloroform reacts with acetone.
So, the correct answer is Option C.
Note: Chloretone is a sedative, preservative, hypnotic or a weak local anaesthetic almost similar to chloral hydrate. Being an anesthetic, it mainly possesses effects that are related to the halothane and isoflurane. There are certain harmful effects of chloretone also which generally include the high toxicity to the liver, also causes irritation to the skin and even behaves as a severe eye irritant.
Complete step by step solution:
Chloretone or chlorobutanol was synthesized for the first time in 1881 by a German chemist namely Conrad Willgerodt. The IUPAC name of chloretone is 1,1,1-Trichloro-2-methyl propan-2-ol. The reaction of acetone with chloroform in the presence of sodium or potassium hydroxide leads to the formation of chloretone. Actually, the anions present in the reactants i.e. \[C{H_3}C{O^ - }\] and \[CC{l_3}^ - \] reacts and form bonds with the cations i.e. \[{H^ + }\] and \[C{H_3}^ + \] respectively, which as a result, leads to the formation of chloretone. It may also undergo sublimation or recrystallization for further purification. The chemical equation for the formation of chloretone has been depicted below:
$C{H_3}COC{H_3} + CC{l_3}\xrightarrow{{KOH}}{C_4}{H_7}C{l_3}O$
It can be written as:

As a result, Chloretone is formed when chloroform reacts with acetone.
So, the correct answer is Option C.
Note: Chloretone is a sedative, preservative, hypnotic or a weak local anaesthetic almost similar to chloral hydrate. Being an anesthetic, it mainly possesses effects that are related to the halothane and isoflurane. There are certain harmful effects of chloretone also which generally include the high toxicity to the liver, also causes irritation to the skin and even behaves as a severe eye irritant.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




