
How many chiral carbons does 2,4-dimethyl pentane have?
Answer
552.3k+ views
Hint: To determine the answer of this question you should know about what is chiral carbon and chiral compound. The chiral compounds are asymmetric in nature where the carbon atom which is attached with four different types of groups and atoms are known as chiral carbon.
Complete step by step answer:
The chiral carbons are defined as the atom which are bonded to four different atoms or the groups. The compound is known as the chiral compound. The chiral compound is tetrahedral in nature and the chiral carbon is $s{p^3}$ hybridized.
The chiral compounds are optically active in nature as they can rotate the plane polarized light in the right hand side or left hand side. The optically active compounds are those which can rotate the plane polarized light either in clockwise or anticlockwise. When the rotation is toward clockwise that it is R configuration and when the rotation is towards anticlockwise than it is S configuration.
The given compound is 2,4-dimethyl pentane.
The structure is shown below.
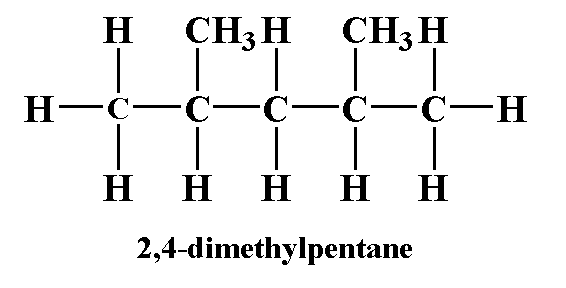
The above structure is drawn using chemdraw.
Here, you can see that every $C{H_3}$ carbon is joined to three hydrogen atoms. The $C{H_2}$ carbon is joined with two hydrogen atoms and in CH carbon is joined with two methyl groups.
So, you can see that no carbon atom is attached with four different atoms or groups.
Therefore, 2,4-dimethyl pentane does not contain any chiral carbon.
Note: Meso compounds are exceptions for the chiral compounds as they do possess multiple chiral centers but usually they are achiral in nature. The compound with double bonds does not contain chiral carbon as they cannot form four double bonds.
Complete step by step answer:
The chiral carbons are defined as the atom which are bonded to four different atoms or the groups. The compound is known as the chiral compound. The chiral compound is tetrahedral in nature and the chiral carbon is $s{p^3}$ hybridized.
The chiral compounds are optically active in nature as they can rotate the plane polarized light in the right hand side or left hand side. The optically active compounds are those which can rotate the plane polarized light either in clockwise or anticlockwise. When the rotation is toward clockwise that it is R configuration and when the rotation is towards anticlockwise than it is S configuration.
The given compound is 2,4-dimethyl pentane.
The structure is shown below.

The above structure is drawn using chemdraw.
Here, you can see that every $C{H_3}$ carbon is joined to three hydrogen atoms. The $C{H_2}$ carbon is joined with two hydrogen atoms and in CH carbon is joined with two methyl groups.
So, you can see that no carbon atom is attached with four different atoms or groups.
Therefore, 2,4-dimethyl pentane does not contain any chiral carbon.
Note: Meso compounds are exceptions for the chiral compounds as they do possess multiple chiral centers but usually they are achiral in nature. The compound with double bonds does not contain chiral carbon as they cannot form four double bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




