
What is the chemical formula of sodium carbonate?
A. \[Na(C{{O}_{3}})\]
B. \[Na{{(C{{O}_{3}})}_{2}}\]
C. \[N{{a}_{2}}(C{{O}_{3}})\]
D. \[N{{a}_{2}}{{(C{{O}_{3}})}_{3}}\]
Answer
603.3k+ views
Hint: In sodium carbonate (other names are washing soda, soda ash or soda crystals), the charge on the sodium cation is +1, the charge on the carbonate anion is -2. So, balance and construct the chemical formula of sodium carbonate with the help of the respective valencies of the ions.
Complete step by step answer:
The valency of a complex ion is the same as the value of the charge of the ion.
Carbonate (\[C{{O}_{3}}^{2-}\]) has a charge of -2, so valency is 2. Valency of sodium (\[N{{a}^{+}}\]) is 1.
Hence, as per crossover rule, crossing valency over:
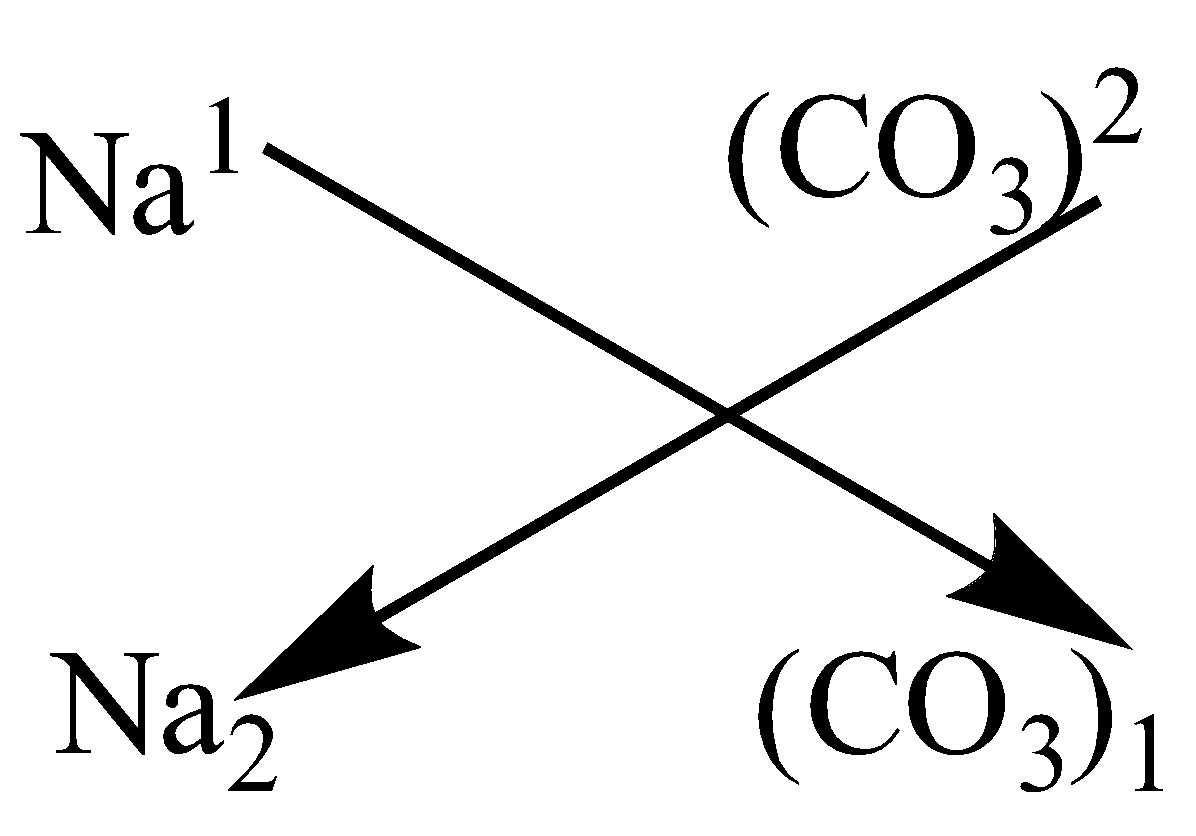
So, the charge is swapped and maintained accordingly. The chemical formula of sodium carbonate is \[N{{a}_{2}}(C{{O}_{3}})\].
Therefore, the correct option is C.
Additional information:
Sodium carbonate- disodium salt of carbonic acid. It exists in three forms of hydrates:
\[N{{a}_{2}}C{{O}_{3}}.\text{10}{{H}_{2}}O\](sodium carbonate decahydrate)
\[N{{a}_{2}}C{{O}_{3}}.7{{H}_{2}}O\](sodium carbonate heptahydrate)
\[N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O\](sodium carbonate monohydrate)
The given compound in the question is the anhydrous salt (salt without any water molecule). Another name for this anhydrous salt is calcined soda. It is manufactured in the last step of the Solvay process when sodium hydrogen carbonate is calculated.
Note: The chemical formula of an ionic compound or a covalent compound tells us the ratio of the elements present in it.
The valency of an atom or ion is the number of electrons it shares, loses or gains during a chemical reaction to attain stability that is the number of bonds it forms with the other atoms.
Complete step by step answer:
The valency of a complex ion is the same as the value of the charge of the ion.
Carbonate (\[C{{O}_{3}}^{2-}\]) has a charge of -2, so valency is 2. Valency of sodium (\[N{{a}^{+}}\]) is 1.
Hence, as per crossover rule, crossing valency over:

So, the charge is swapped and maintained accordingly. The chemical formula of sodium carbonate is \[N{{a}_{2}}(C{{O}_{3}})\].
Therefore, the correct option is C.
Additional information:
Sodium carbonate- disodium salt of carbonic acid. It exists in three forms of hydrates:
\[N{{a}_{2}}C{{O}_{3}}.\text{10}{{H}_{2}}O\](sodium carbonate decahydrate)
\[N{{a}_{2}}C{{O}_{3}}.7{{H}_{2}}O\](sodium carbonate heptahydrate)
\[N{{a}_{2}}C{{O}_{3}}.{{H}_{2}}O\](sodium carbonate monohydrate)
The given compound in the question is the anhydrous salt (salt without any water molecule). Another name for this anhydrous salt is calcined soda. It is manufactured in the last step of the Solvay process when sodium hydrogen carbonate is calculated.
Note: The chemical formula of an ionic compound or a covalent compound tells us the ratio of the elements present in it.
The valency of an atom or ion is the number of electrons it shares, loses or gains during a chemical reaction to attain stability that is the number of bonds it forms with the other atoms.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




