
What is the chemical formula for lead (IV) oxide?
Answer
504.3k+ views
Hint: Lead oxide is really harmful to human beings and it is also poisonous in nature. Lead oxide is widely used in matches, dyes and explosives as well. The (IV) in lead (IV) oxide suggests that the oxidation number of lead in lead (IV) oxide is 4, that is IV.
Complete answer:
The chemical formula for lead (IV) oxide is given by \[Pb{{O}_{2}}\] , in which there are two atoms of oxygen ( \[O\] ) and one atom of lead ( \[Pb\] ).
The (IV) in lead (IV) oxide is the oxidation number of lead, the oxidation number is positive so it is +4. One atom of oxygen takes up two electrons and thus two atoms of oxygen will take up four electrons, therefore one lead atom has to give four electrons to form a stable product.
Therefore the oxidation number of lead in lead (IV) oxide is +4.
The structural formula of lead (IV) oxide is shown below.
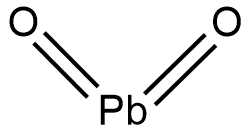
Lead (IV) oxide is also commonly known as lead oxide, plumbic oxide or anhydrous plumbic acid. The IUPAC name of lead (IV) oxide is dioxoplumbane. The bond between lead and oxygen atom shows both ionic and covalent character. Thus, the ionic character gives the compound its lattice structure and the covalent character gives the compound its low melting point and insolubility in water.
Note:
Lead oxide is a dark brown and odourless crystalline powder which is nearly insoluble in water. It exists in two crystalline forms, orthorhombic and tetragonal, the orthorhombic one is identified in nature as a rare mineral scrutinyite. It is a strong oxidizing agent which is used in manufacturing of matches, dyes and other chemicals. It is vastly used in positive plates of lead acid batteries.
Complete answer:
The chemical formula for lead (IV) oxide is given by \[Pb{{O}_{2}}\] , in which there are two atoms of oxygen ( \[O\] ) and one atom of lead ( \[Pb\] ).
The (IV) in lead (IV) oxide is the oxidation number of lead, the oxidation number is positive so it is +4. One atom of oxygen takes up two electrons and thus two atoms of oxygen will take up four electrons, therefore one lead atom has to give four electrons to form a stable product.
Therefore the oxidation number of lead in lead (IV) oxide is +4.
The structural formula of lead (IV) oxide is shown below.

Lead (IV) oxide is also commonly known as lead oxide, plumbic oxide or anhydrous plumbic acid. The IUPAC name of lead (IV) oxide is dioxoplumbane. The bond between lead and oxygen atom shows both ionic and covalent character. Thus, the ionic character gives the compound its lattice structure and the covalent character gives the compound its low melting point and insolubility in water.
Note:
Lead oxide is a dark brown and odourless crystalline powder which is nearly insoluble in water. It exists in two crystalline forms, orthorhombic and tetragonal, the orthorhombic one is identified in nature as a rare mineral scrutinyite. It is a strong oxidizing agent which is used in manufacturing of matches, dyes and other chemicals. It is vastly used in positive plates of lead acid batteries.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




