
What is the chemical formula for copper (II) hydroxide?
Answer
525.6k+ views
Hint: Copper (II) hydroxide is a bluish-green solid compound made by adding sodium hydroxide to a dilute solution of copper (II) sulphate pentahydrate. The cupric ion comes from copper (II) sulphate and NaOH produces hydroxide ion. Knowing the oxidation states of the atom, we can write the chemical formula.
Complete answer:
In naming the inorganic chemical compounds, a systematic method is recommended by the International Union of Pure and Applied Chemistry (IUPAC) and that is known as IUPAC nomenclature.
According to this nomenclature method, ionic compounds are named by writing the cation name first followed by the name of its anionic counterpart. The positive ion retains its element name and to denote the number of elements present, the prefix mono-, di-, tri-, etc. is used.
Some metal elements can possess more than one charge, the naming of such compounds is done by writing the charge using roman numerals in parentheses immediately following the metal ion’s name.
Let us consider the case of copper (II) hydroxide. It is an ionic inorganic compound made up of copper metal and a hydroxide ion. It is a slightly strong base. To write its name, we need to find out the charge present on the elements.
The charge possessed by copper is written in the parenthesis, that is, 2 and we know that hydroxide ion carries a negative charge. Thus, by using the criss-cross method we can write the formula of the compound as given below:
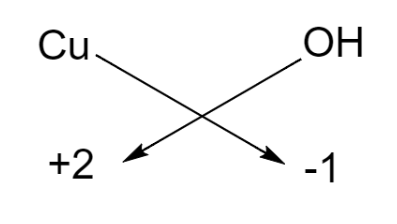
Hence, the chemical formula for copper (II) hydroxide is $\text{Cu}{{\left( \text{OH} \right)}_{2}}$.
Note:
Instead of writing Roman numerals, the different types of ions can also be represented by specific words. Such as $\text{Cu}{{\left( \text{OH} \right)}_{2}}$ is also known as cupric hydroxide and $\text{Cu}\left( \text{OH} \right)$ is known as cuprous hydroxide. -ous suffix is used for lower oxidation state while -ic is used for higher oxidation state.
Complete answer:
In naming the inorganic chemical compounds, a systematic method is recommended by the International Union of Pure and Applied Chemistry (IUPAC) and that is known as IUPAC nomenclature.
According to this nomenclature method, ionic compounds are named by writing the cation name first followed by the name of its anionic counterpart. The positive ion retains its element name and to denote the number of elements present, the prefix mono-, di-, tri-, etc. is used.
Some metal elements can possess more than one charge, the naming of such compounds is done by writing the charge using roman numerals in parentheses immediately following the metal ion’s name.
Let us consider the case of copper (II) hydroxide. It is an ionic inorganic compound made up of copper metal and a hydroxide ion. It is a slightly strong base. To write its name, we need to find out the charge present on the elements.
The charge possessed by copper is written in the parenthesis, that is, 2 and we know that hydroxide ion carries a negative charge. Thus, by using the criss-cross method we can write the formula of the compound as given below:

Hence, the chemical formula for copper (II) hydroxide is $\text{Cu}{{\left( \text{OH} \right)}_{2}}$.
Note:
Instead of writing Roman numerals, the different types of ions can also be represented by specific words. Such as $\text{Cu}{{\left( \text{OH} \right)}_{2}}$ is also known as cupric hydroxide and $\text{Cu}\left( \text{OH} \right)$ is known as cuprous hydroxide. -ous suffix is used for lower oxidation state while -ic is used for higher oxidation state.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




