
Bring out the following conversions:
Acetylene to Acetaldehyde
Answer
527.1k+ views
Hint: As we know that acetylene is also known as ethyne and it is the simplest alkyne molecule in organic chemistry. The chemical formula of acetylene is ${{C}_{2}}{{H}_{2}}$ . It is a colourless gas which is widely used as fuel. So here we have to show the formation of acetaldehyde from acetylene.
Complete answer:
There are various methods to convert acetylene to acetaldehyde among which the most preferred reaction conversion is given below:-
-Hydration of acetylene (ethyne) in the presence of mercuric sulphate ($HgS{{O}_{4}}$) and sulphuric acid (${{H}_{2}}S{{O}_{4}}$) at high temperature:-
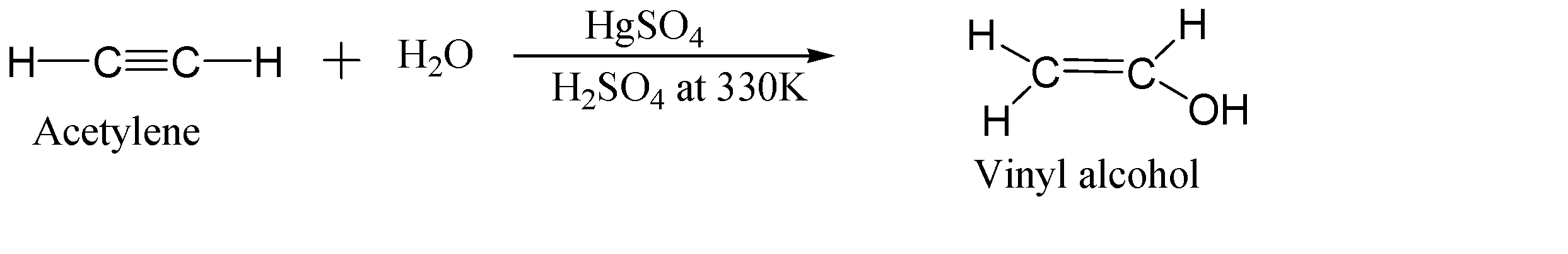
In this step, the acetylene compound gets hydrated by oxidation of pi-bond. This reaction is carried out in the presence of mercuric sulphate with dilute sulphuric acid at very high temperature. When acetylene gets hydrated, it forms vinyl alcohol which is very unstable and tends to tautomerize.
-Keto-enol tautomerization:-
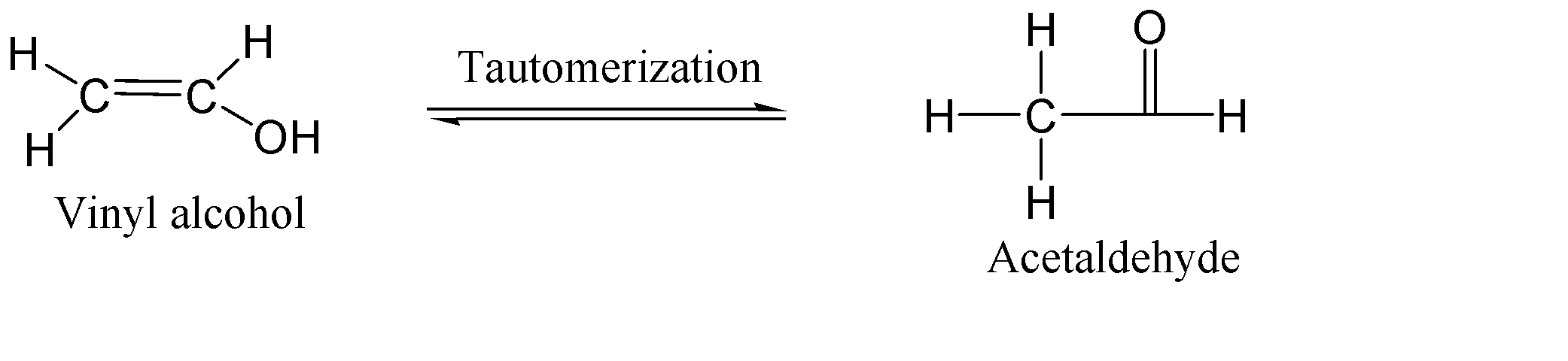
Keto–enol tautomerism is a chemical equilibrium in organic chemistry that exists between a keto form of compound (a ketone or an aldehyde) and an enol form of compound (an alcohol). Both of these forms are said to be tautomers of each other.
Here vinyl alcohol is the enol form which is unstable and tends to tautomerize to its keto form which is ethanol or acetaldehyde. Here the hydrogen of alcohol part (-OH) shifts towards other carbon of the double bond and this double bond shifts its electron density between carbon and oxygen which forms a carbonyl group.
-This way acetylene is converted to acetaldehyde.
Note:
-The other two methods are given below:-
(a) Use of acetylene hydratase enzyme which catalyzes the hydration of acetylene and yields acetaldehyde.
(b) Use $LiAl{{H}_{4}}$ to give ethane from ethyne and then react with chlorine in the presence of sunlight to produce chloroethane. Then react this with aqueous potassium hydroxide to release ethanol and finally use PCC reagent to form acetaldehyde.
Complete answer:
There are various methods to convert acetylene to acetaldehyde among which the most preferred reaction conversion is given below:-
-Hydration of acetylene (ethyne) in the presence of mercuric sulphate ($HgS{{O}_{4}}$) and sulphuric acid (${{H}_{2}}S{{O}_{4}}$) at high temperature:-

In this step, the acetylene compound gets hydrated by oxidation of pi-bond. This reaction is carried out in the presence of mercuric sulphate with dilute sulphuric acid at very high temperature. When acetylene gets hydrated, it forms vinyl alcohol which is very unstable and tends to tautomerize.
-Keto-enol tautomerization:-

Keto–enol tautomerism is a chemical equilibrium in organic chemistry that exists between a keto form of compound (a ketone or an aldehyde) and an enol form of compound (an alcohol). Both of these forms are said to be tautomers of each other.
Here vinyl alcohol is the enol form which is unstable and tends to tautomerize to its keto form which is ethanol or acetaldehyde. Here the hydrogen of alcohol part (-OH) shifts towards other carbon of the double bond and this double bond shifts its electron density between carbon and oxygen which forms a carbonyl group.
-This way acetylene is converted to acetaldehyde.
Note:
-The other two methods are given below:-
(a) Use of acetylene hydratase enzyme which catalyzes the hydration of acetylene and yields acetaldehyde.
(b) Use $LiAl{{H}_{4}}$ to give ethane from ethyne and then react with chlorine in the presence of sunlight to produce chloroethane. Then react this with aqueous potassium hydroxide to release ethanol and finally use PCC reagent to form acetaldehyde.
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

State and prove Bernoullis theorem class 11 physics CBSE

Actinoid contraction is more than lanthanoid contraction class 11 chemistry CBSE

The transition element that has lowest enthalpy of class 11 chemistry CBSE

Can anyone list 10 advantages and disadvantages of friction

State the laws of reflection of light




