
How many bridging oxygen atoms are present in ${P_4}{O_{10}}$?
(A) 6
(B) 4
(C) 2
(D) 5
Answer
570.3k+ views
Hint: Phosphorus pentoxide, ${P_4}{O_{10}}$, is a dimer of the compound phosphorus (V) oxide, ${P_2}{O_5}$. It is a covalent compound. Phosphorus is basically a pentavalent element and it reacts with oxygen to form oxides such as ${P_2}{O_3}$ and ${P_4}{O_{10}}$. Both of these oxides of phosphorus exist as dimer that means these compounds are formed by a combination of two molecules of that compound. The compound ${P_4}{O_{10}}$ is a very good dehydrating agent.
Complete step by step solution: To find out the number of bridging oxygen atoms in ${P_4}{O_{10}}$, we need to first understand what does a bridging atom means. Further, we need to draw and understand the chemical structure of ${P_4}{O_{10}}$.
In ${P_4}{O_{10}}$, there are 4 phosphorus atoms and 10 oxygen atoms. Each phosphorus atom in ${P_4}{O_{10}}$ is linked to 4 oxygen atoms.
The diagram given below is the structure of ${P_4}{O_{10}}$.
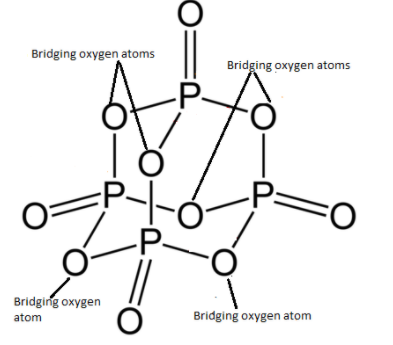
As you can see, the phosphorus atoms and oxygen atoms bonded to form a closed cage like structure. There are six $P - O - P$ bonds and each oxygen atom undergoes $s{p^3}$ hybridisation. There are 4 $P = O$ where hybridisation of oxygen is $s{p^2}$. As we are aware of the fact that each oxygen will have two lone pairs of electrons. Therefore, the total number of lone pairs of electrons in ${P_4}{O_{10}}$ are 20.
As there are six $P - O - P$ bonds present in ${P_4}{O_{10}}$, these six oxygen atoms combine two tetrahedrons together. These 6 oxygen atoms are known as bridging oxygen atoms.
Therefore, six bridging oxygen atoms are present in ${P_4}{O_{10}}$.
Hence, the correct answer is option (A).
Note: In ${P_4}{O_{10}}$, oxygen is bridging atom which help to combine two molecules of ${P_2}{O_5}$ to form a dimer. Here, we can say that the monomer is ${P_2}{O_5}$. The oxygen atom is a bridging atom. Students should not confuse the term ‘bridging atom’ with ‘ligand’. Ligand is the term used in coordination chemistry. A ligand can be an ion or molecule and it binds the central metal atom or ion to form coordination complexes. It can be anion or cation or sometimes sit can be a neutral molecule. The examples of ligands include $N{O^ + }$, $N{H_3}$, ${H_2}O$. The bridging ligand will bound more than one metal atom in the coordination complex.
Complete step by step solution: To find out the number of bridging oxygen atoms in ${P_4}{O_{10}}$, we need to first understand what does a bridging atom means. Further, we need to draw and understand the chemical structure of ${P_4}{O_{10}}$.
In ${P_4}{O_{10}}$, there are 4 phosphorus atoms and 10 oxygen atoms. Each phosphorus atom in ${P_4}{O_{10}}$ is linked to 4 oxygen atoms.
The diagram given below is the structure of ${P_4}{O_{10}}$.

As you can see, the phosphorus atoms and oxygen atoms bonded to form a closed cage like structure. There are six $P - O - P$ bonds and each oxygen atom undergoes $s{p^3}$ hybridisation. There are 4 $P = O$ where hybridisation of oxygen is $s{p^2}$. As we are aware of the fact that each oxygen will have two lone pairs of electrons. Therefore, the total number of lone pairs of electrons in ${P_4}{O_{10}}$ are 20.
As there are six $P - O - P$ bonds present in ${P_4}{O_{10}}$, these six oxygen atoms combine two tetrahedrons together. These 6 oxygen atoms are known as bridging oxygen atoms.
Therefore, six bridging oxygen atoms are present in ${P_4}{O_{10}}$.
Hence, the correct answer is option (A).
Note: In ${P_4}{O_{10}}$, oxygen is bridging atom which help to combine two molecules of ${P_2}{O_5}$ to form a dimer. Here, we can say that the monomer is ${P_2}{O_5}$. The oxygen atom is a bridging atom. Students should not confuse the term ‘bridging atom’ with ‘ligand’. Ligand is the term used in coordination chemistry. A ligand can be an ion or molecule and it binds the central metal atom or ion to form coordination complexes. It can be anion or cation or sometimes sit can be a neutral molecule. The examples of ligands include $N{O^ + }$, $N{H_3}$, ${H_2}O$. The bridging ligand will bound more than one metal atom in the coordination complex.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

What organs are located on the left side of your body class 11 biology CBSE

Draw a diagram of nephron and explain its structur class 11 biology CBSE




