
Borazole contains ______ bonds
(A) 9 Sigma, 6 Pi
(B) 6 Sigma, 9 Pi
(C) 12 Sigma, 3 Pi
(D) 15 Sigma, 0 Pi
Answer
548.4k+ views
Hint: Borazole is an inorganic and cyclic compound. In order to solve this question, we need to draw the structure of borazine and identify the sigma and pi bonds. It has a structure similar to benzene but with alternating boron and nitrogen atoms instead of carbon.
Complete Step by step solution
Sigma bond in chemistry is the strongest type of covalent bond. Sigma bond is created when atomic orbitals undergo head-on overlapping. The electrons forming sigma bonds are known as sigma electrons. In a structure, all single bonds are usually sigma bonds.
Pi bonds are generally weaker than the sigma bonds. They are formed by sideways overlap of atomic orbitals. They are formed along directions perpendicular to the internuclear axis. For a double bond in a structure, there is one sigma bond and one pi bond. For a triple bond in a structure, there is one sigma bond and two pi bonds.
Borazole, which is also known as borazine, has the chemical formula $ {B_3}{N_3}{H_6} $ .
The structure of borazole is as follows,
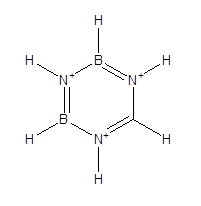
The structure of borazine has B-H sigma bonds, 3 N-H sigma bonds and 6 B-N sigma bonds that equals to a total 12 sigma bonds. The number of pi bonds is 3 and they are between B and N atoms. Therefore, borazole contains 12 sigma bonds and 3 pi bonds.
Hence, the correct answer is option C.
Note
Borazole and benzene are isostructural and isoelectronic. Hence for this reason, the other name of borazole is inorganic benzene. Benzene and borazine both are colourless liquids.
Complete Step by step solution
Sigma bond in chemistry is the strongest type of covalent bond. Sigma bond is created when atomic orbitals undergo head-on overlapping. The electrons forming sigma bonds are known as sigma electrons. In a structure, all single bonds are usually sigma bonds.
Pi bonds are generally weaker than the sigma bonds. They are formed by sideways overlap of atomic orbitals. They are formed along directions perpendicular to the internuclear axis. For a double bond in a structure, there is one sigma bond and one pi bond. For a triple bond in a structure, there is one sigma bond and two pi bonds.
Borazole, which is also known as borazine, has the chemical formula $ {B_3}{N_3}{H_6} $ .
The structure of borazole is as follows,

The structure of borazine has B-H sigma bonds, 3 N-H sigma bonds and 6 B-N sigma bonds that equals to a total 12 sigma bonds. The number of pi bonds is 3 and they are between B and N atoms. Therefore, borazole contains 12 sigma bonds and 3 pi bonds.
Hence, the correct answer is option C.
Note
Borazole and benzene are isostructural and isoelectronic. Hence for this reason, the other name of borazole is inorganic benzene. Benzene and borazine both are colourless liquids.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




