
Borax structure contains?
A. Two $B{{O}_{4}}$ groups and two $B{{O}_{3}}$ groups
B. Four$B{{O}_{4}}$ groups only
C. Four $B{{O}_{3}}$ groups only
D. Three $B{{O}_{4}}$ groups and one $B{{O}_{3}}$ group
Answer
524.7k+ views
Hint: Borax is a compound of boron it has a structure having tetra nuclear units. Borax is a salt that contains water of crystallization. It is an important compound of boron. It gives a famous borax bead test on subjection with heat to form transparent beads like glass. They consist of sodium Meta borate and boric anhydride.
Complete answer:
Borax is an important compound of boron. It has a formula $N{{a}_{2}}[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}].8{{H}_{2}}O$ and thus also called as sodium tetraborate. Its structure consist of tetra nuclear units of the formula${{\left[ {{B}_{4}}{{O}_{5}}{{(OH)}_{4}} \right]}^{2-}}$
The structure of borax consists of an arrangement with 4 boron atoms such that 2 boron atoms form 3 – coordinate bonds, while the other 2 boron atoms form 4 – coordinate bonds. Thus the structure gives rise to the formation of two groups of boron and oxygen arrangement. The arrangement where 2 boron atoms are in the form of tetrahedral atoms having two $B{{O}_{4}}$groups, and the other arrangement where the other 2 boron atoms have a planar arrangement with 3 oxygen atoms as $B{{O}_{3}}$ groups. The structure of borax is as follows:
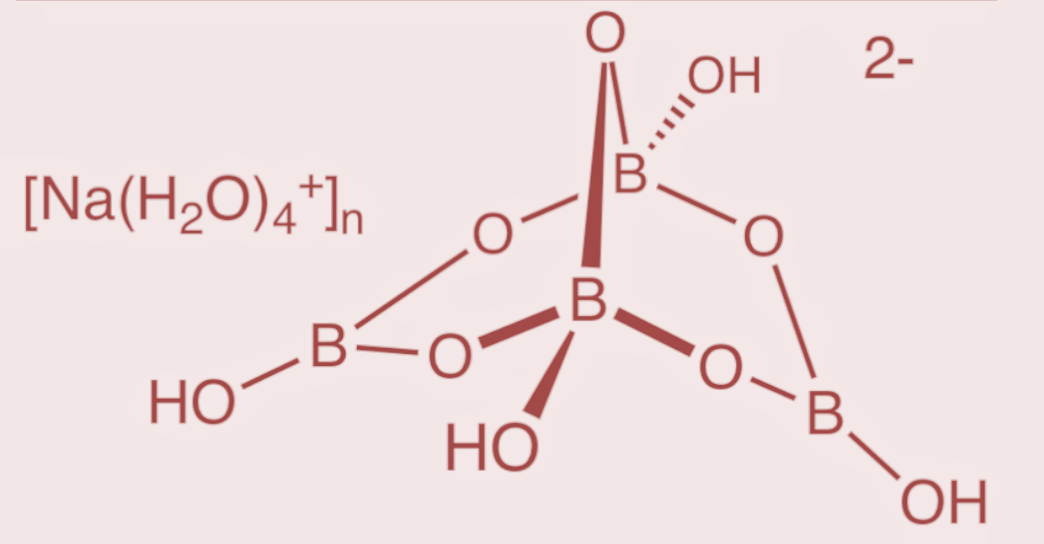
Hence, the borax structure contains two $B{{O}_{4}}$ groups and two $B{{O}_{3}}$groups.
So, option A is correct.
Note:
Borax is one of the most useful compounds of boron. It is used in preparing antiseptic medicinal soaps, for water softening, manufacturing borosilicate glass, soldering etc. It gives a known borax bead test that when subjected to heating it forms glass like beads that are important and useful in qualitative analysis of radicals.
Complete answer:
Borax is an important compound of boron. It has a formula $N{{a}_{2}}[{{B}_{4}}{{O}_{5}}{{(OH)}_{4}}].8{{H}_{2}}O$ and thus also called as sodium tetraborate. Its structure consist of tetra nuclear units of the formula${{\left[ {{B}_{4}}{{O}_{5}}{{(OH)}_{4}} \right]}^{2-}}$
The structure of borax consists of an arrangement with 4 boron atoms such that 2 boron atoms form 3 – coordinate bonds, while the other 2 boron atoms form 4 – coordinate bonds. Thus the structure gives rise to the formation of two groups of boron and oxygen arrangement. The arrangement where 2 boron atoms are in the form of tetrahedral atoms having two $B{{O}_{4}}$groups, and the other arrangement where the other 2 boron atoms have a planar arrangement with 3 oxygen atoms as $B{{O}_{3}}$ groups. The structure of borax is as follows:

Hence, the borax structure contains two $B{{O}_{4}}$ groups and two $B{{O}_{3}}$groups.
So, option A is correct.
Note:
Borax is one of the most useful compounds of boron. It is used in preparing antiseptic medicinal soaps, for water softening, manufacturing borosilicate glass, soldering etc. It gives a known borax bead test that when subjected to heating it forms glass like beads that are important and useful in qualitative analysis of radicals.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




