
How many bonds do carbon forms in neutral compounds?
Answer
554.1k+ views
Hint: First of all we should know about carbon, its properties and bond formation with other compounds. Carbon is a chemical element whose atomic number is six with the symbol as C. It is a non-metal that generally shows tetravalency, which means that the maximum bond formed by carbon is only four.
Complete step-by-step answer:About carbon atom:-
General characteristic of carbon atom:-
-It is abundant, common.
-It generally forms strong covalent bonds.
-It has four valence electrons, hence it will form four bonds with other compounds.
-It has a variety of shapes.
-It generally forms bonds with multiple elements.
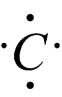
Chemical properties of carbon atom are as follows:-
-Its chemical formula is C.
-Oxidation: It generally combines with the oxygen to produce carbon dioxide and carbon monoxide.
-Reactivity: It does not react or dissolve in water or acids.
-Chains of Atoms: It has the ability to make long chains of atoms.
Uses of carbon atom:-
-The human body comprises 18% of the total carbon atom.
-It is also found in sugar, glucose, proteins etc.
-Its isotope is diamond which is generally used in making jewelry.
-Amorphous carbon is used to make inks and paints.
-Graphite is an isotope of carbon which is generally used as the lead in pencils.
-Carbon dating is one of the most important uses of carbon atoms.
-Carbon is widely used in industries.
Carbon generally forms four covalent bonds with neutral atoms, because of having four valence electrons in its outermost shell or orbit.
Note: Carbon has an exceptional ability to form bonds with a large variety of bonds. It generally shows anomalous behavior due to various properties such as high ionization energy, high electrification, exceptional size and absence of d-orbitals.
Complete step-by-step answer:About carbon atom:-
General characteristic of carbon atom:-
-It is abundant, common.
-It generally forms strong covalent bonds.
-It has four valence electrons, hence it will form four bonds with other compounds.
-It has a variety of shapes.
-It generally forms bonds with multiple elements.
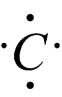
Chemical properties of carbon atom are as follows:-
-Its chemical formula is C.
-Oxidation: It generally combines with the oxygen to produce carbon dioxide and carbon monoxide.
-Reactivity: It does not react or dissolve in water or acids.
-Chains of Atoms: It has the ability to make long chains of atoms.
Uses of carbon atom:-
-The human body comprises 18% of the total carbon atom.
-It is also found in sugar, glucose, proteins etc.
-Its isotope is diamond which is generally used in making jewelry.
-Amorphous carbon is used to make inks and paints.
-Graphite is an isotope of carbon which is generally used as the lead in pencils.
-Carbon dating is one of the most important uses of carbon atoms.
-Carbon is widely used in industries.
Carbon generally forms four covalent bonds with neutral atoms, because of having four valence electrons in its outermost shell or orbit.
Note: Carbon has an exceptional ability to form bonds with a large variety of bonds. It generally shows anomalous behavior due to various properties such as high ionization energy, high electrification, exceptional size and absence of d-orbitals.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




