
What is the bond polarity of \[N{H_3}\]?
A) \[N{H_3}\] is a non-polar molecule
B) \[N{H_3}\] is a polar molecular
C) \[N - H\] bond is polar
D) Both B and C
Answer
513k+ views
Hint: We also have to know that the polarity comes when Hydrogen atoms are attached to an electronegative species. A molecule is said to be polar when the distribution of electrons between the atoms that are covalently bonded to it is not polar. On the other hand A nonpolar molecule is the one in which electrons are distributed equally.
Complete answer:
We will going to see the structure, hybridization and geometry before answering this question
We can draw the structure of ammonia as,
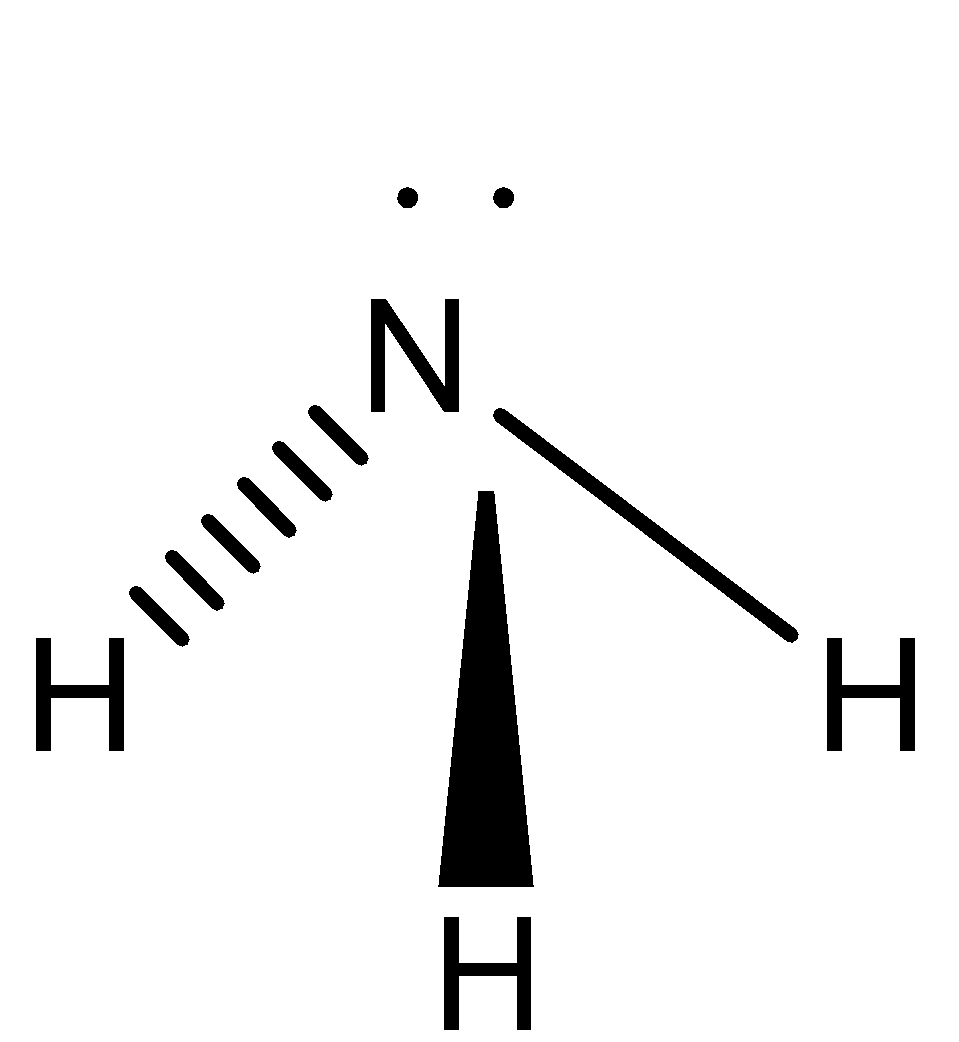
We also remember that the hybridization of ammonia is \[s{p^3}\]
Molecular Geometry: Pyramidal
As we know that every \[N - H\] bond is polar in ammonia since the electronegativities of both Nitrogen and Hydrogen are different. The molecular geometry of ammonia is not symmetrical due to which it will not have 0 dipole moment and polarities will not be cancelled.
\[N\] will have partial negative charge while H atom will have partial positive charge. \[N\] is much more electronegative than the H atom, due to which ammonia turns out to be a polar molecule.
There are \[3N - H\] bonds in ammonia and one lone pair of atoms on \[N\].
The distance between \[N - H\] bond is \[101.7pm\] and the angle between \[HNH\] is \[107.8^\circ \]
Example of Polar molecule: \[N{H_3}\], \[{H_2}O\]
Example of Non Polar molecule: \[C{H_3},NaCl\]
Option A) this is an incorrect option as ammonia is not non polar as discussed above.
Option B) this option is right as Ammonia is a polar molecule which we have discussed but we have other options too, so look at all the options
Option C) this option is right as \[N - H\] bond is polar due to the variant electronegativity between H and N, but we will look at other options too.
Option D) this is a correct option as it contains both the options which are right.
Note:
We have to remember that the bond polarity basically tells about the polarity of bond. Since ammonia is a polar molecule it will have polar bonds as well, which is \[N - H\] bond in this case.
Complete answer:
We will going to see the structure, hybridization and geometry before answering this question
We can draw the structure of ammonia as,

We also remember that the hybridization of ammonia is \[s{p^3}\]
Molecular Geometry: Pyramidal
As we know that every \[N - H\] bond is polar in ammonia since the electronegativities of both Nitrogen and Hydrogen are different. The molecular geometry of ammonia is not symmetrical due to which it will not have 0 dipole moment and polarities will not be cancelled.
\[N\] will have partial negative charge while H atom will have partial positive charge. \[N\] is much more electronegative than the H atom, due to which ammonia turns out to be a polar molecule.
There are \[3N - H\] bonds in ammonia and one lone pair of atoms on \[N\].
The distance between \[N - H\] bond is \[101.7pm\] and the angle between \[HNH\] is \[107.8^\circ \]
Example of Polar molecule: \[N{H_3}\], \[{H_2}O\]
Example of Non Polar molecule: \[C{H_3},NaCl\]
Option A) this is an incorrect option as ammonia is not non polar as discussed above.
Option B) this option is right as Ammonia is a polar molecule which we have discussed but we have other options too, so look at all the options
Option C) this option is right as \[N - H\] bond is polar due to the variant electronegativity between H and N, but we will look at other options too.
Option D) this is a correct option as it contains both the options which are right.
Note:
We have to remember that the bond polarity basically tells about the polarity of bond. Since ammonia is a polar molecule it will have polar bonds as well, which is \[N - H\] bond in this case.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




