
Why bond angle in water is less than that of ammonia although their geometries are distorted tetrahedral?
Answer
594.6k+ views
Hint: The concept of inter mixing of different atomic orbitals and then redistribution of energy to give new orbitals of symmetrical orientation in shape, identical shape, and equivalent energy of orbitals. The new orbitals formed are known as hybrid orbital and this process is known as hybridization. Some molecules do not show their geometrical structure follows hybridization.
Complete step by step solution:
A simple theory based on the repulsive interactions of the electron pairs in the valence shell of an atom is valence shell electron pair repulsion (VSEPR) theory. This theory provides a simple procedure to predict the shapes of molecules.
Assumptions of VSEPR theory:
Paired electrons in the valence shell of the center atom remain as lone pair, other than that unpaired electrons in the central atom form a bond with the unpaired electrons with surrounding atoms.
Bond pair and lone pair in the molecule repel each other.
Based on the number of bond pairs and lone pairs surrounding the central atom, geometry and shape of the molecule changes.
Bond pairs repulsions around the central atom should be minimum than lone pairs repulsions.
There are three types of repulsion in the molecule are lp-lp, lp-bp and bp-bp.
The order of magnitude of repulsive forces are :lp-lp>lp-bp>bp-bp.
The given ammonia and water molecules are involved in $s{{p}^{3}}$ and expected structure to be tetrahedral. But there are one lone pair in ammonia and two lone pairs in water molecules that cause a distorted tetrahedral structure.
Because of one lone pair in ammonia molecule, the distorted tetrahedral become pyramid with bond angle is ${{107}^{o}}$ and there is more lp-lp repulsion in water molecule the distorted tetrahedral become V-shape with bond angle ${{104.5}^{o}}$ .
Hence, there are more lp-lp repulsions in water molecule, therefore bond angle is less than that of ammonia although both geometries are distorted tetrahedral.
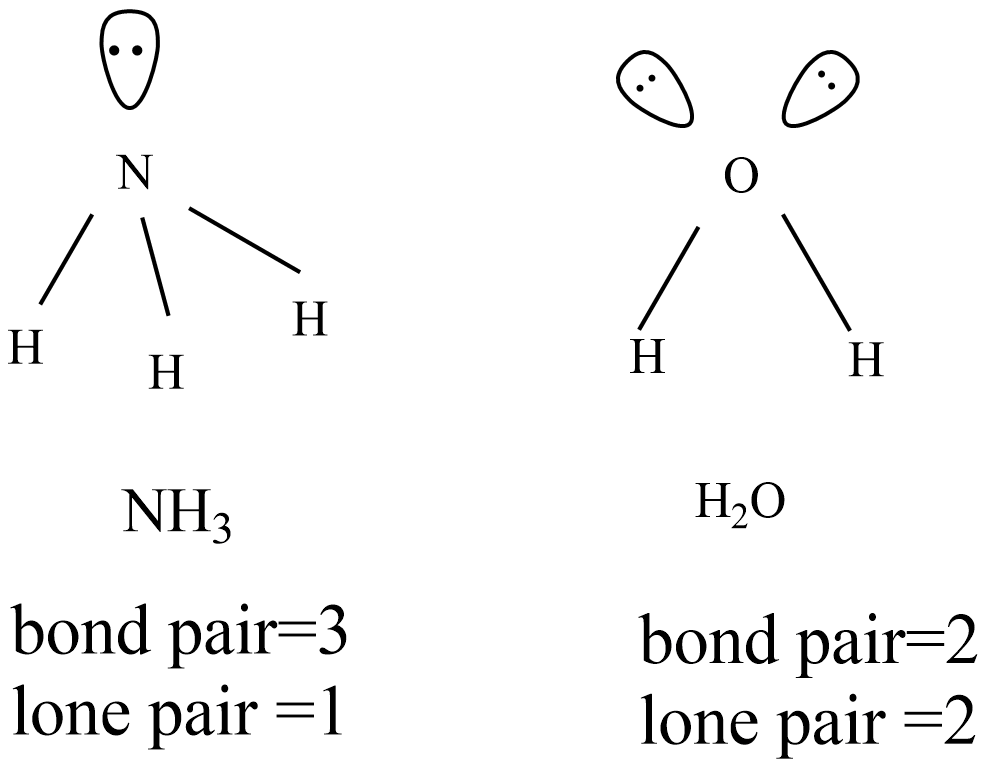
Note: One of the main limitations of VSEPR theory is about bond angle deviations. This theory predicts molecular geometry, but cannot predict the shape of transition metal compounds. In d-block heavier elements experience the stereochemical inert pair effect.
Complete step by step solution:
A simple theory based on the repulsive interactions of the electron pairs in the valence shell of an atom is valence shell electron pair repulsion (VSEPR) theory. This theory provides a simple procedure to predict the shapes of molecules.
Assumptions of VSEPR theory:
Paired electrons in the valence shell of the center atom remain as lone pair, other than that unpaired electrons in the central atom form a bond with the unpaired electrons with surrounding atoms.
Bond pair and lone pair in the molecule repel each other.
Based on the number of bond pairs and lone pairs surrounding the central atom, geometry and shape of the molecule changes.
Bond pairs repulsions around the central atom should be minimum than lone pairs repulsions.
There are three types of repulsion in the molecule are lp-lp, lp-bp and bp-bp.
The order of magnitude of repulsive forces are :lp-lp>lp-bp>bp-bp.
The given ammonia and water molecules are involved in $s{{p}^{3}}$ and expected structure to be tetrahedral. But there are one lone pair in ammonia and two lone pairs in water molecules that cause a distorted tetrahedral structure.
Because of one lone pair in ammonia molecule, the distorted tetrahedral become pyramid with bond angle is ${{107}^{o}}$ and there is more lp-lp repulsion in water molecule the distorted tetrahedral become V-shape with bond angle ${{104.5}^{o}}$ .
Hence, there are more lp-lp repulsions in water molecule, therefore bond angle is less than that of ammonia although both geometries are distorted tetrahedral.
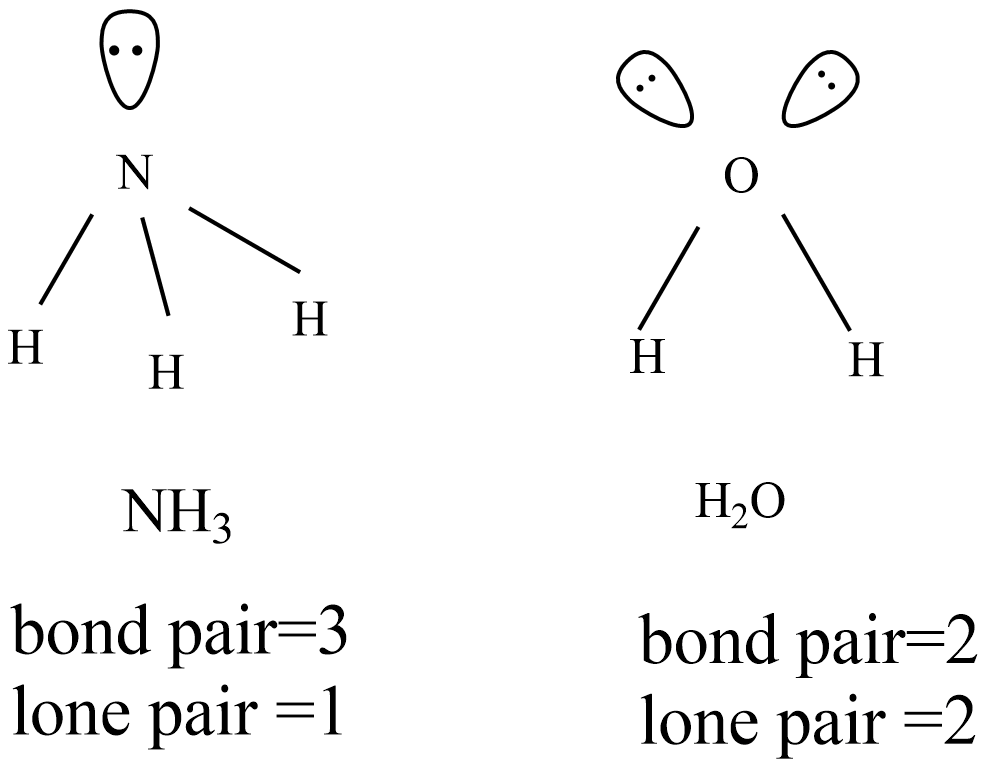
Note: One of the main limitations of VSEPR theory is about bond angle deviations. This theory predicts molecular geometry, but cannot predict the shape of transition metal compounds. In d-block heavier elements experience the stereochemical inert pair effect.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




