
$B{{F}_{3}}$ is a planar molecule whereas $N{{F}_{3}}$ is pyramidal because:
(A) B - F bond is more polar than N - F bond
(B) Boron atom is bigger than nitrogen atom
(C) Nitrogen is more electronegative than boron
(D) $B{{F}_{3}}$ has no lone pair but $N{{F}_{3}}$ has a lone pair of electrons.
Answer
588.6k+ views
Hint: Write the electronic configuration of boron and nitrogen atoms. Draw the structure of boron fluoride and nitrogen trifluoride. Take a look at the types of electron-pairs present in the molecule and then determine the correct reason.
Complete step by step solution:
- Boron has atomic number 5 and nitrogen has atomic number 7. Their electronic configuration is given as follows,
\[{}^{5}B=1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}\]
\[{}^{7}N=1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
- Boron is forming three covalent bonds with fluorine so it is $s{{p}^{2}}$ hybridized due to the presence of three electrons present in the outermost shell. On excitation of boron atom, the electronic configuration will be ${}^{5}B=1{{s}^{2}}2{{s}^{1}}2{{p}^{2}}$ and so, three orbitals of three fluorine atoms will axially overlap with three $s{{p}^{2}}$ hybridized orbitals of boron to form boron trifluoride.
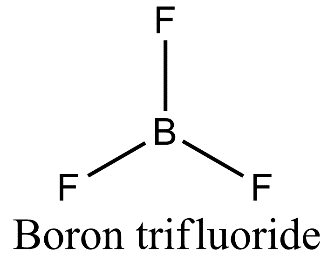
- Nitrogen is also forming three covalent bonds with fluorine but when nitrogen atom is excited, the electronic configuration is ${}^{7}N=1{{s}^{2}}2{{s}^{1}}2{{p}^{4}}$ and so, nitrogen undergoes $s{{p}^{3}}$ hybridization in which one of the $s{{p}^{3}}$ hybrid orbital will have a pair of electrons and three other orbitals will have one electron each. These three orbitals having a single electron will axially overlap with three orbitals of three fluorine atoms to form nitrogen trifluoride.
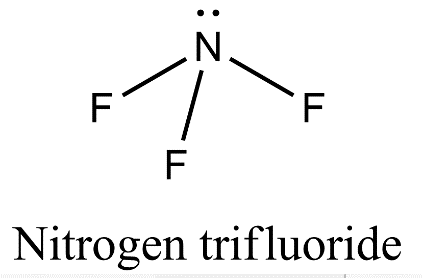
- Now, since nitrogen contains one lone pair of electrons there will be lone pair- bond pair repulsion which will lead to distortion of trigonal planar geometry. Therefore, nitrogen trifluoride will have pyramidal geometry.
- In case of boron trifluoride, there is vacant 2p orbital and no lone pair of electrons and so there is only bond pair- bond pair repulsion giving rise to trigonal planar geometry.
- Therefore, $B{{F}_{3}}$ is a planar molecule whereas $N{{F}_{3}}$ is pyramidal because $B{{F}_{3}}$ has no lone pair but $N{{F}_{3}}$ has a lone pair of electrons.
- Hence, option (D) is the correct answer.
Note: Remember lone pair - lone pair repulsion is greater than lone pair- bond pair repulsion which is greater than bond pair- bond pair repulsion. Due to the presence of lone pairs, there is distortion of geometry.
Complete step by step solution:
- Boron has atomic number 5 and nitrogen has atomic number 7. Their electronic configuration is given as follows,
\[{}^{5}B=1{{s}^{2}}2{{s}^{2}}2{{p}^{1}}\]
\[{}^{7}N=1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}\]
- Boron is forming three covalent bonds with fluorine so it is $s{{p}^{2}}$ hybridized due to the presence of three electrons present in the outermost shell. On excitation of boron atom, the electronic configuration will be ${}^{5}B=1{{s}^{2}}2{{s}^{1}}2{{p}^{2}}$ and so, three orbitals of three fluorine atoms will axially overlap with three $s{{p}^{2}}$ hybridized orbitals of boron to form boron trifluoride.

- Nitrogen is also forming three covalent bonds with fluorine but when nitrogen atom is excited, the electronic configuration is ${}^{7}N=1{{s}^{2}}2{{s}^{1}}2{{p}^{4}}$ and so, nitrogen undergoes $s{{p}^{3}}$ hybridization in which one of the $s{{p}^{3}}$ hybrid orbital will have a pair of electrons and three other orbitals will have one electron each. These three orbitals having a single electron will axially overlap with three orbitals of three fluorine atoms to form nitrogen trifluoride.

- Now, since nitrogen contains one lone pair of electrons there will be lone pair- bond pair repulsion which will lead to distortion of trigonal planar geometry. Therefore, nitrogen trifluoride will have pyramidal geometry.
- In case of boron trifluoride, there is vacant 2p orbital and no lone pair of electrons and so there is only bond pair- bond pair repulsion giving rise to trigonal planar geometry.
- Therefore, $B{{F}_{3}}$ is a planar molecule whereas $N{{F}_{3}}$ is pyramidal because $B{{F}_{3}}$ has no lone pair but $N{{F}_{3}}$ has a lone pair of electrons.
- Hence, option (D) is the correct answer.
Note: Remember lone pair - lone pair repulsion is greater than lone pair- bond pair repulsion which is greater than bond pair- bond pair repulsion. Due to the presence of lone pairs, there is distortion of geometry.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




