
Benzene was first synthesized by passing acetylene through a red hot tube (Berthelot). This is an example of condensation polymerization.
A. True
B. False
Answer
601.5k+ views
Hint: We know that, Condensation polymerization generally involves a repetitive condensation reaction between two bi-functional or trifunctional mono-meric units. These polymerization reactions may result in the loss of some simple molecules as water, alcohol, hydrogen chloride etc and lead to the formation of high molecular mass condensation polymers.
Complete step by step solution:
Berthelot was a scientist and one day he found that acetylene passed through a red-hot tube and gave benzene. Benzene is very stable to oxidants and in fact resistance to oxidation is a strong characteristic of the benzene ring. This is an example of additional polymerization.
The reaction is stated below-
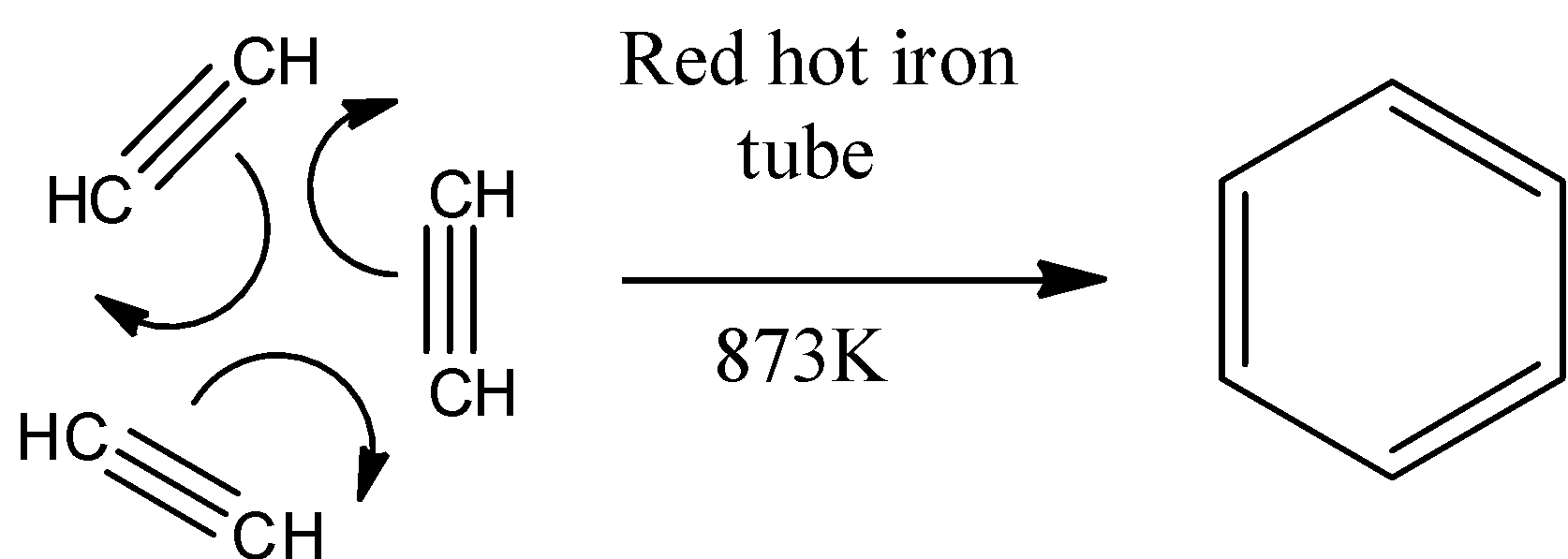
This process is also called cyclic polymerization. The temperature required to carry out the reaction is 873K. There are three molecules of ethyne that undergo cyclic polymerization form benzene. This is called an additional reaction because the molecules of the same monomer or different monomers add together on a large scale to form a polymer in this type of polymerization reaction. The monomers used are unsaturated compounds, e.g.: alkenes, alkadienes and their derivatives. This mode of polymerization leads to an increase in chain length and chain growth can take place through the formation of either free radicals or ionic species. Therefore, the above statement is (b) False.
Note: We should know about the resonance energy of the benzene. It plays an important role in the stabilization of aromatic compounds. It is the difference in energy between actual structure of compound and most stable resonating structure. The resonance energy of benzene is 150.325$\text{Jmo}{{\text{l}}^{-1}}$.
Complete step by step solution:
Berthelot was a scientist and one day he found that acetylene passed through a red-hot tube and gave benzene. Benzene is very stable to oxidants and in fact resistance to oxidation is a strong characteristic of the benzene ring. This is an example of additional polymerization.
The reaction is stated below-

This process is also called cyclic polymerization. The temperature required to carry out the reaction is 873K. There are three molecules of ethyne that undergo cyclic polymerization form benzene. This is called an additional reaction because the molecules of the same monomer or different monomers add together on a large scale to form a polymer in this type of polymerization reaction. The monomers used are unsaturated compounds, e.g.: alkenes, alkadienes and their derivatives. This mode of polymerization leads to an increase in chain length and chain growth can take place through the formation of either free radicals or ionic species. Therefore, the above statement is (b) False.
Note: We should know about the resonance energy of the benzene. It plays an important role in the stabilization of aromatic compounds. It is the difference in energy between actual structure of compound and most stable resonating structure. The resonance energy of benzene is 150.325$\text{Jmo}{{\text{l}}^{-1}}$.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




