
$BeC{l_2} + LiAl{H_4} \to X + LiCl + AlC{l_3}$ :
A. $X$ is lithium hydride
B. $X$ is $Be{H_2}$
C. $X$ is $BeC{l_2}.2{H_2}O$
D. $X$ is $LiH$
Answer
563.4k+ views
Hint: The components of the reactants are important for product formation after the reaction has taken place. The cations and the anions present in the reactants are involved in the process and product formation requires a balance according to that of the reactants.
Complete step-by-step answer: The chloride ions which are present in the $BeC{l_2}$ molecule is removed from the molecule during the reaction process. The removal of the chloride then results in the substitution by the hydrogen residues. The formation of $Be{H_2}$ takes place when the hydrogen from the $LiAl{H_4}$ molecule is removed and then it replaces the chloride residues. The bond formation of $Be{H_2}$ forms the continuous chain structure forming hexagonal crystalline forms with the heavy density of molecules. The two molecules of $Be{H_2}$ together forms a bonded structure due to the presence of the empty orbitals in $Be$ which is known as a $3 - $ centred $ - 2 - $ electron bonds $\left( {3c-2e} \right)$. The structure forms a combined continuous structure. This type of bonding pattern is known as a banana bond and it helps in the formation of a polymeric structure. This polymeric structure offers stability to the compound as the monomeric form is unstable. The polymeric structure is insoluble in the solvents and it forms a colourless compound at the end of the reaction process. The structure of two $Be{H_2}$ bonds formed together is given below showing the banana bonded structure.
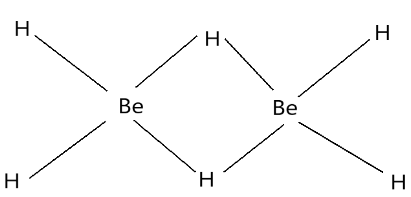
It is visible that on two sides the multimeric form of this compound is possible resulting in the structure which is crystalline and insoluble in solvents.
Hence the correct option is (B).
Note: The stable structure needs to be formed as an end product of the reaction. This is why if substitution of the elements takes place during the reaction, the compounds will be formed according to the higher stability order.
Complete step-by-step answer: The chloride ions which are present in the $BeC{l_2}$ molecule is removed from the molecule during the reaction process. The removal of the chloride then results in the substitution by the hydrogen residues. The formation of $Be{H_2}$ takes place when the hydrogen from the $LiAl{H_4}$ molecule is removed and then it replaces the chloride residues. The bond formation of $Be{H_2}$ forms the continuous chain structure forming hexagonal crystalline forms with the heavy density of molecules. The two molecules of $Be{H_2}$ together forms a bonded structure due to the presence of the empty orbitals in $Be$ which is known as a $3 - $ centred $ - 2 - $ electron bonds $\left( {3c-2e} \right)$. The structure forms a combined continuous structure. This type of bonding pattern is known as a banana bond and it helps in the formation of a polymeric structure. This polymeric structure offers stability to the compound as the monomeric form is unstable. The polymeric structure is insoluble in the solvents and it forms a colourless compound at the end of the reaction process. The structure of two $Be{H_2}$ bonds formed together is given below showing the banana bonded structure.

It is visible that on two sides the multimeric form of this compound is possible resulting in the structure which is crystalline and insoluble in solvents.
Hence the correct option is (B).
Note: The stable structure needs to be formed as an end product of the reaction. This is why if substitution of the elements takes place during the reaction, the compounds will be formed according to the higher stability order.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




