
Baeyer’s reagent is?
A.Alkaline potassium permanganate
B.Acidified potassium permanganate
C.Neutral potassium permanganate
D.Alkaline potassium manganate
Answer
596.1k+ views
Hint:You should know that it is a great oxidizing agent. It is also used to differentiate between saturated and unsaturated hydrocarbons. Now try to recall the Baeyer’s test and try to figure it out by yourself.
Complete step by step answer:
-On a very general basis, Baeyer’s test is a laboratory test to identify the presence of double bonds in a given unsaturated compound.
-Baeyer's test is basically a test for unsaturation. In this test addition of bromine is done to a double bond, which results in the rapid decolourization of bromine water or bromine dissolved in carbon tetrachloride, is taken as a test for unsaturation.
Let us understand in detail.
Baeyer’s reagent is a very cold dilute solution of alkaline potassium permanganate. It is a purple or light violet color solution. Baeyer’s reagent is a strong oxidizing reagent which is used to identify the presence of double or triple bonds in a hydrocarbon. Thus it indicates the unsaturation of hydrocarbon compounds. For example, when purple colour reagent is added to ethylene, it obtains a colorless solution of ethane-1,2-diol.
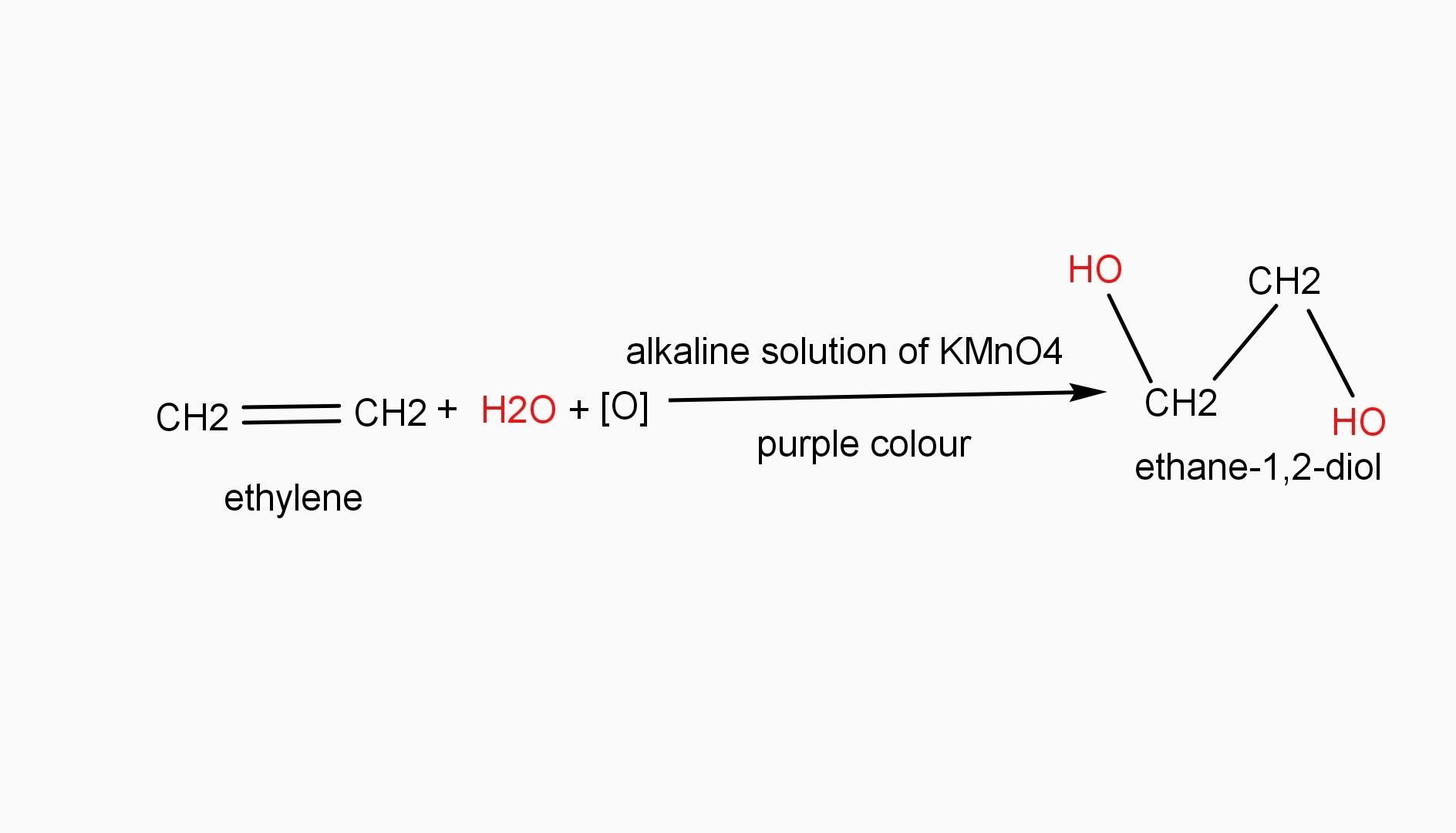
Therefore, Baeyer’s reagent is alkaline potassium permanganate. Hence, option A is the required answer.
Note:
Generally, when potassium permanganate is applied to your skin, it kills germs by releasing oxygen when it meets compounds in your skin. It also acts as an astringent, which is a drying agent.
Complete step by step answer:
-On a very general basis, Baeyer’s test is a laboratory test to identify the presence of double bonds in a given unsaturated compound.
-Baeyer's test is basically a test for unsaturation. In this test addition of bromine is done to a double bond, which results in the rapid decolourization of bromine water or bromine dissolved in carbon tetrachloride, is taken as a test for unsaturation.
Let us understand in detail.
Baeyer’s reagent is a very cold dilute solution of alkaline potassium permanganate. It is a purple or light violet color solution. Baeyer’s reagent is a strong oxidizing reagent which is used to identify the presence of double or triple bonds in a hydrocarbon. Thus it indicates the unsaturation of hydrocarbon compounds. For example, when purple colour reagent is added to ethylene, it obtains a colorless solution of ethane-1,2-diol.

Therefore, Baeyer’s reagent is alkaline potassium permanganate. Hence, option A is the required answer.
Note:
Generally, when potassium permanganate is applied to your skin, it kills germs by releasing oxygen when it meets compounds in your skin. It also acts as an astringent, which is a drying agent.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




