
(a)Write the products formed when \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]reacts with the following reagents:
(i) \[{\text{HCN}}\]
(ii) \[{{\text{H}}_{\text{2}}}{\text{N - OH}}\]
(iii) \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]in the presence of dilute\[{\text{NaOH}}\]
(b) Give the simple chemical tests to distinguish between the following pair of compounds
(i) Propanal and propanone
Answer
598.5k+ views
Hint- we must remember that the \[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\] known as acetaldehyde and has aldehyde as the functional group. The carbon-oxygen double bond of \[{\text{ - CHO}}\]is polarised due to higher electronegativity of oxygen relative to carbon. Therefore we can understand the carbonyl oxygen is a nucleophilic (Lewis base) centre and the carbonyl carbon is an electrophilic (Lewis acid) and which can undergo nucleophilic addition reactions.
We must understand that the propanal is an aldehyde and propanone is a ketone, so we need to find a test to distinguish between aldehyde and ketone. Therefore we can use Iodoform test to inspect the presence of carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or alcohols with the structure\[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\].
Complete step by step solution:-
(a) (i) when we react aldehydes and ketones with hydrogen cyanide $(HCN)$ which yield cyanohydrins. So in the given question, acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) reacts with hydrogen cyanide \[{\text{HCN}}\] to give 2-hydroxypropanenitrile as product. We can represent the reactions as
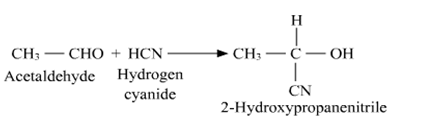
(ii) When we reacts acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) with hydroxylamine (\[{{\text{H}}_{\text{2}}}{\text{N - OH}}\]) to give acetaldoxime (oxime) as a product.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}{\text{ + N}}{{\text{H}}_{\text{2}}}{\text{OH}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{ - CH = NOH + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
(iii) Also the reaction of acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) with acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) in the presence of dilute\[{\text{NaOH}}\], we know this is a type of aldol reaction by which we obtained 3-hydroxybutanal as product. Further, we heat the reaction mixture, we get aldol condensation product (but-2-enal).
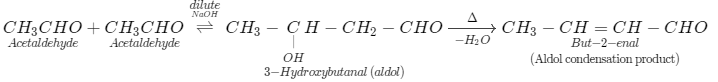
(b) We can use the Iodoform test to distinguish propanal and propanone. We must understand that when we treat this compound with iodine in the presence of base like\[{\text{NaOH}}\], we get a pale yellow precipitate of triiodomethane (iodoform) for carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or we get alcohols with the structure\[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\]. Now we can distinguish, propanone has \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]structure gives positive result with iodoform while propanal do not contain any such structure gives negative result.
\[
\begin{array}{*{20}{l}}
{{\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{ + }}{{\text{I}}_{\text{2}}}{\text{ + NaOH }} \to {\text{CH}}{{\text{I}}_{\text{3}}}{\text{ (iodoform) + C}}{{\text{H}}_{\text{3}}}{\text{COONa + NaOH}}} \\
{\text{Propanone}} \\
{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO + }}{{\text{I}}_{\text{2}}}{\text{ + NaOH }} \to {\text{No reaction}} \\
{\text{Propanal}} \\
\end{array} \\
\\
\]
Note- (a) we must know that all the reactions of acetaldehyde with corresponding reactants is a result of polarised C-O bonds in the aldehyde.
(b) We must look for carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or alcohols with the structure \[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\]in order to carry out iodoform test.
We must understand that the propanal is an aldehyde and propanone is a ketone, so we need to find a test to distinguish between aldehyde and ketone. Therefore we can use Iodoform test to inspect the presence of carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or alcohols with the structure\[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\].
Complete step by step solution:-
(a) (i) when we react aldehydes and ketones with hydrogen cyanide $(HCN)$ which yield cyanohydrins. So in the given question, acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) reacts with hydrogen cyanide \[{\text{HCN}}\] to give 2-hydroxypropanenitrile as product. We can represent the reactions as

(ii) When we reacts acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) with hydroxylamine (\[{{\text{H}}_{\text{2}}}{\text{N - OH}}\]) to give acetaldoxime (oxime) as a product.
\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}{\text{ + N}}{{\text{H}}_{\text{2}}}{\text{OH}} \to {\text{C}}{{\text{H}}_{\text{3}}}{\text{ - CH = NOH + }}{{\text{H}}_{\text{2}}}{\text{O}}\]
(iii) Also the reaction of acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) with acetaldehyde (\[{\text{C}}{{\text{H}}_{\text{3}}}{\text{CHO}}\]) in the presence of dilute\[{\text{NaOH}}\], we know this is a type of aldol reaction by which we obtained 3-hydroxybutanal as product. Further, we heat the reaction mixture, we get aldol condensation product (but-2-enal).
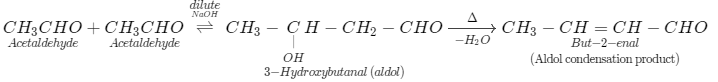
(b) We can use the Iodoform test to distinguish propanal and propanone. We must understand that when we treat this compound with iodine in the presence of base like\[{\text{NaOH}}\], we get a pale yellow precipitate of triiodomethane (iodoform) for carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or we get alcohols with the structure\[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\]. Now we can distinguish, propanone has \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]structure gives positive result with iodoform while propanal do not contain any such structure gives negative result.
\[
\begin{array}{*{20}{l}}
{{\text{C}}{{\text{H}}_{\text{3}}}{\text{COC}}{{\text{H}}_{\text{3}}}{\text{ + }}{{\text{I}}_{\text{2}}}{\text{ + NaOH }} \to {\text{CH}}{{\text{I}}_{\text{3}}}{\text{ (iodoform) + C}}{{\text{H}}_{\text{3}}}{\text{COONa + NaOH}}} \\
{\text{Propanone}} \\
{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO + }}{{\text{I}}_{\text{2}}}{\text{ + NaOH }} \to {\text{No reaction}} \\
{\text{Propanal}} \\
\end{array} \\
\\
\]
Note- (a) we must know that all the reactions of acetaldehyde with corresponding reactants is a result of polarised C-O bonds in the aldehyde.
(b) We must look for carbonyl compounds with the structure \[{\text{R - CO - C}}{{\text{H}}_{\text{3}}}\]or alcohols with the structure \[{\text{R - CH}}\left( {{\text{OH}}} \right){\text{ - C}}{{\text{H}}_{\text{3}}}\]in order to carry out iodoform test.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




