
(A)Which metal exists in liquid state at room temperature?
(B)Write electronic configuration of nitrogen and chlorine atoms.
(C)Show the formation of sodium chloride by the transfer of electrons between nitrogen and chlorine atoms.
Answer
591.3k+ views
Hint: For (A): This is a transition group element and is very less reactive. It also has an inner filled f orbital which does ineffective shielding of outermost electrons. Also being a liquid makes it useful for thermometers.
For (B): First check its atomic number then fill the electrons according to their increasing energy levels.
For (C): Sodium loses an electron to form a cation and chlorine receives an electron to form an anion.
Complete answer:
(A)At room temperature mercury (Hg) exists in the form of liquid. It has a melting point of 234.32 K. In the periodic table it is present in the 6th period and 12th group and has an atomic number of 80. It is a transition group element.
It has an electronic configuration of: \[[Xe]4{f^{14}}5{d^{10}}6{s^2}\]
It is an exceptional case since other elements surrounding it exist in solid state only. It is also much less reactive than other elements of its group (cadmium and zinc). Its capacity to conduct heat and electricity is also very low as compared to other elements of its group.
We all know that most of the metals share their valence electrons with its surrounding atoms and obtain their solid state. But in mercury the nucleus attracts its valence electrons more tightly than other atoms and thus the bond between two mercury atoms is very weak as compared to other metals.
(B)First of all we will check the atomic numbers of these 2 elements.
For nitrogen (N): Its atomic number is 7 and so it accommodates 7 electrons. So the first 2 electrons will go into 1s orbital (s can accommodate only 2 electrons), then the next 2 in 2s orbital and the remaining 3 in 2p orbital (p can accommodate 6 electrons).
Its electronic configuration will be: $1{s^2}2{s^2}2{p^3}$.
For chlorine (Cl): Its atomic number is 17 and thus it will accommodate 17 electrons. The first 2 electrons will go into 1s orbital, the next 2 in 2s, then the next 6 in 2p, then the next 2 in 3s and the remaining 5 in 3p orbital.
So, its electronic configuration will be: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$.
(C)Sodium chloride consists of sodium and chlorine atoms in their ionic forms. Sodium is present as $N{a^ + }$ and chlorine is present as $C{l^ - }$. Since Na has the tendency to lose one electron and gain stability and chlorine can take an electron to gain stability they form an ionic bond here. In short we can say that Na donates its 1 electron to Cl. This can be shown as:
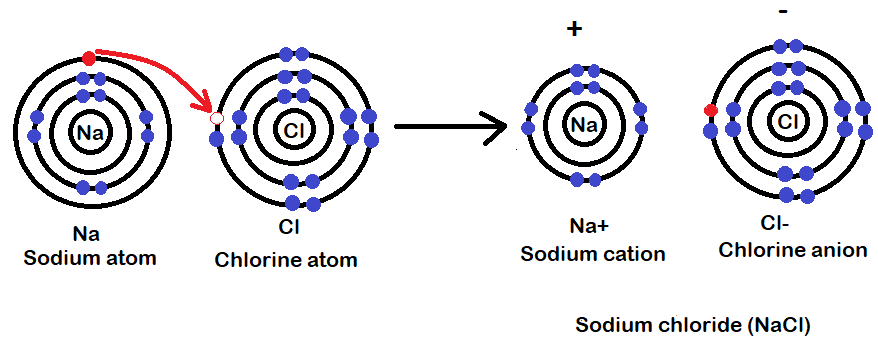
Note:
For (A): In Hg the 6s shell is stable due to presence of a completely filled 4f shell. The nuclear charge which increases the attraction between the 6s shell and nucleus is not shielded properly by the f shell. Thus it has a lower melting point, while Cd and Zn have higher melting point due to absence of filled f orbital.
For (B): The electrons are filled in this sequence of orbitals because the Aufbau principle states that the orbitals with lower energy are filled first. Thus the order of increasing orbital energy will be:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on.
For (C): Sodium can donate an electron because it is a metal and chlorine accepts an electron because it is a non-metal.
For (B): First check its atomic number then fill the electrons according to their increasing energy levels.
For (C): Sodium loses an electron to form a cation and chlorine receives an electron to form an anion.
Complete answer:
(A)At room temperature mercury (Hg) exists in the form of liquid. It has a melting point of 234.32 K. In the periodic table it is present in the 6th period and 12th group and has an atomic number of 80. It is a transition group element.
It has an electronic configuration of: \[[Xe]4{f^{14}}5{d^{10}}6{s^2}\]
It is an exceptional case since other elements surrounding it exist in solid state only. It is also much less reactive than other elements of its group (cadmium and zinc). Its capacity to conduct heat and electricity is also very low as compared to other elements of its group.
We all know that most of the metals share their valence electrons with its surrounding atoms and obtain their solid state. But in mercury the nucleus attracts its valence electrons more tightly than other atoms and thus the bond between two mercury atoms is very weak as compared to other metals.
(B)First of all we will check the atomic numbers of these 2 elements.
For nitrogen (N): Its atomic number is 7 and so it accommodates 7 electrons. So the first 2 electrons will go into 1s orbital (s can accommodate only 2 electrons), then the next 2 in 2s orbital and the remaining 3 in 2p orbital (p can accommodate 6 electrons).
Its electronic configuration will be: $1{s^2}2{s^2}2{p^3}$.
For chlorine (Cl): Its atomic number is 17 and thus it will accommodate 17 electrons. The first 2 electrons will go into 1s orbital, the next 2 in 2s, then the next 6 in 2p, then the next 2 in 3s and the remaining 5 in 3p orbital.
So, its electronic configuration will be: $1{s^2}2{s^2}2{p^6}3{s^2}3{p^5}$.
(C)Sodium chloride consists of sodium and chlorine atoms in their ionic forms. Sodium is present as $N{a^ + }$ and chlorine is present as $C{l^ - }$. Since Na has the tendency to lose one electron and gain stability and chlorine can take an electron to gain stability they form an ionic bond here. In short we can say that Na donates its 1 electron to Cl. This can be shown as:

Note:
For (A): In Hg the 6s shell is stable due to presence of a completely filled 4f shell. The nuclear charge which increases the attraction between the 6s shell and nucleus is not shielded properly by the f shell. Thus it has a lower melting point, while Cd and Zn have higher melting point due to absence of filled f orbital.
For (B): The electrons are filled in this sequence of orbitals because the Aufbau principle states that the orbitals with lower energy are filled first. Thus the order of increasing orbital energy will be:
1s < 2s < 2p < 3s < 3p < 4s < 3d < 4p < 5s < 4d < 5p < 6s < 4f < 5d < 6p < 7s < 5f < 6d < 7p and so on.
For (C): Sodium can donate an electron because it is a metal and chlorine accepts an electron because it is a non-metal.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




