
What atomic or hybrid orbitals make up the sigma bond between \[I\] and \[Cl\] in iodine pentachloride, \[IC{l_5}\]?
Answer
519.3k+ views
Hint: The intermixing of two or more pure atomic orbitals of an atom with almost the same energy to give the same number of identical and degenerate new types of orbitals is known as hybridization. The new orbitals formed are also known as hybrid orbitals.
Formula to find the hybridization of a molecule is given by
\[Hybridisation = Number{\text{ }}of{\text{ }}\sigma {\text{ }}bonds + {\text{ }}Number{\text{ }}of{\text{ }}lone{\text{ }}pairs\]
Complete answer:
If the sum is \[2\] \[ \to \] hybridization − \[sp\]
If the sum is \[3\] \[ \to \] hybridization − \[s{p^2}\]
If the sum is \[4\] \[ \to \] hybridization − \[s{p^3}\]
If the sum is \[5\] \[ \to \] hybridization− \[s{p^3}d\]
If the sum is \[6\] \[ \to \] hybridization− \[s{p^3}{d^2}\]
COMPLETE STEP BY STEP ANSWER-
Lewis dot structure of \[IC{l_5}\]:
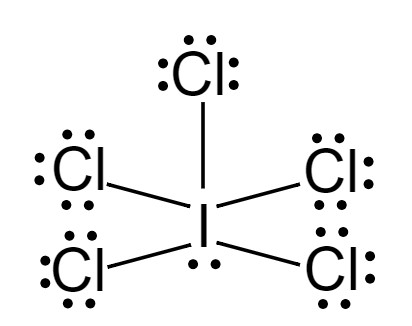
Considering central metal atom, \[I\]
$ Number{\text{ }}of{\text{ }}lone{\text{ }}pairs = 1 \\
Number{\text{ }}of{\text{ }}bond{\text{ }}pairs = 5 \\ $
\[Hybridisation = 1 + 5 = 6\]
Therefore, \[I - Cl\] bond is \[s{p^3}{d^2}\] hybridized.
About \[IC{l_5}\],
Hybridisation-\[s{p^3}{d^2}\]
Geometry- Octahedral
Shape- Square pyramidal
Note:
You can expect bond angles of \[{90^ \circ }\] and \[{180^ \circ }\].
Also note that when forming hybrid orbitals, you use \[n\] atomic orbitals to make \[n\] hybrid orbitals, where \[n\] is the total amount of orbitals. Here, in case of \[IC{l_5}\], \[n = 6\]
Formula to find the hybridization of a molecule is given by
\[Hybridisation = Number{\text{ }}of{\text{ }}\sigma {\text{ }}bonds + {\text{ }}Number{\text{ }}of{\text{ }}lone{\text{ }}pairs\]
Complete answer:
If the sum is \[2\] \[ \to \] hybridization − \[sp\]
If the sum is \[3\] \[ \to \] hybridization − \[s{p^2}\]
If the sum is \[4\] \[ \to \] hybridization − \[s{p^3}\]
If the sum is \[5\] \[ \to \] hybridization− \[s{p^3}d\]
If the sum is \[6\] \[ \to \] hybridization− \[s{p^3}{d^2}\]
COMPLETE STEP BY STEP ANSWER-
Lewis dot structure of \[IC{l_5}\]:

Considering central metal atom, \[I\]
$ Number{\text{ }}of{\text{ }}lone{\text{ }}pairs = 1 \\
Number{\text{ }}of{\text{ }}bond{\text{ }}pairs = 5 \\ $
\[Hybridisation = 1 + 5 = 6\]
Therefore, \[I - Cl\] bond is \[s{p^3}{d^2}\] hybridized.
About \[IC{l_5}\],
Hybridisation-\[s{p^3}{d^2}\]
Geometry- Octahedral
Shape- Square pyramidal
Note:
You can expect bond angles of \[{90^ \circ }\] and \[{180^ \circ }\].
Also note that when forming hybrid orbitals, you use \[n\] atomic orbitals to make \[n\] hybrid orbitals, where \[n\] is the total amount of orbitals. Here, in case of \[IC{l_5}\], \[n = 6\]
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




