
At 143 K, the reaction of \[Xe{F_4}\] with \[{O_2}{F_2}\] produces a Xenon compound Y. The total number of lone pair(s) electrons present on the whole molecule of Y is
Answer
598.5k+ views
Hint-The compound \[Xe{F_4}\] on reaction with \[{O_2}{F_2}\] prouces \[Xe{F_6}\].Therefore we can find the lone pair of electrons by easily drawing the lewis structure of the compound.
Formula used:
We must remember that, the number of Lone pair electrons = Total valence electrons – number of bonding electrons
Complete step by step answer:
We must understand that xenon tetrafluoride is the first binary noble compound to be discovered.Its chemical formula is \[Xe{F_4}\]. Its geometrical shape is square planar and it consists of two lone pairs of electrons in its Xenon center when Xenon forms covalent bonds with four F and also 12 lone pairs of electrons on F atoms. Hence we can represent the Lewis structure of \[Xe{F_4}\] as given below.
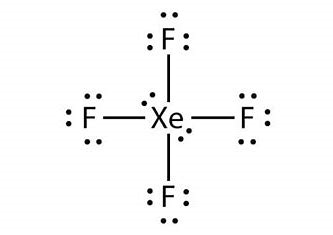
When we react this compound \[Xe{F_4}\] with\[{O_2}{F_2}\], at a temperature of 143 K it results in the formation of\[Xe{F_6}\]. So, we can understand the compound Y formed is Xenon Hexafluoride (XeF6). The geometry of the compound Xenon hexafluoride (\[Xe{F_6}\]) is considered to be distorted octahedral.
$Xe{F_4} + {O_2}{F_2} \to Xe{F_6} + {O_2}$
The Lewis structure of the compound \[Xe{F_6}\]
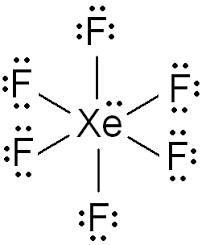
Now, we are asked to find the total number of lone pairs of electrons in compound Y which is \[Xe{F_6}\]. For this, we need to know the Lewis structure of the compound. Lone pair of electrons is where the two electrons are not involved in any bonding. When we know the number of valence electrons and the number of bonding electrons, it becomes easy to find the number of lone pairs of electrons.
Total number valence electrons = valence electrons of Xe + 4 (valence electrons of F)
= 8 + 6 x 7 = 50 valence electrons
Here as Xenon is less electronegative compared to fluorine, it gets in the center surrounded by 6 F atoms. Now, 6 covalent bonds are created between the atoms involving 12 electrons.
Hence we can understand that the number of bonding electrons is 12.
\[Xe{F_6}\]We know that,
Number of lone pair of electrons = Total valence electrons – number of bonding electrons
= 50 – 12
= 38
Therefore, there are 19 pairs of lone electrons with 38 electrons (a pair has two electrons).
Note: Always remember, the lone pair of electrons of Fluorine is not considered as it satisfies the octet rule in many cases. While Xenon, because of its d- orbital is able to violate this octet rule.
Formula used:
We must remember that, the number of Lone pair electrons = Total valence electrons – number of bonding electrons
Complete step by step answer:
We must understand that xenon tetrafluoride is the first binary noble compound to be discovered.Its chemical formula is \[Xe{F_4}\]. Its geometrical shape is square planar and it consists of two lone pairs of electrons in its Xenon center when Xenon forms covalent bonds with four F and also 12 lone pairs of electrons on F atoms. Hence we can represent the Lewis structure of \[Xe{F_4}\] as given below.

When we react this compound \[Xe{F_4}\] with\[{O_2}{F_2}\], at a temperature of 143 K it results in the formation of\[Xe{F_6}\]. So, we can understand the compound Y formed is Xenon Hexafluoride (XeF6). The geometry of the compound Xenon hexafluoride (\[Xe{F_6}\]) is considered to be distorted octahedral.
$Xe{F_4} + {O_2}{F_2} \to Xe{F_6} + {O_2}$
The Lewis structure of the compound \[Xe{F_6}\]

Now, we are asked to find the total number of lone pairs of electrons in compound Y which is \[Xe{F_6}\]. For this, we need to know the Lewis structure of the compound. Lone pair of electrons is where the two electrons are not involved in any bonding. When we know the number of valence electrons and the number of bonding electrons, it becomes easy to find the number of lone pairs of electrons.
Total number valence electrons = valence electrons of Xe + 4 (valence electrons of F)
= 8 + 6 x 7 = 50 valence electrons
Here as Xenon is less electronegative compared to fluorine, it gets in the center surrounded by 6 F atoms. Now, 6 covalent bonds are created between the atoms involving 12 electrons.
Hence we can understand that the number of bonding electrons is 12.
\[Xe{F_6}\]We know that,
Number of lone pair of electrons = Total valence electrons – number of bonding electrons
= 50 – 12
= 38
Therefore, there are 19 pairs of lone electrons with 38 electrons (a pair has two electrons).
Note: Always remember, the lone pair of electrons of Fluorine is not considered as it satisfies the octet rule in many cases. While Xenon, because of its d- orbital is able to violate this octet rule.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




