
Assertion: The IUPAC name of
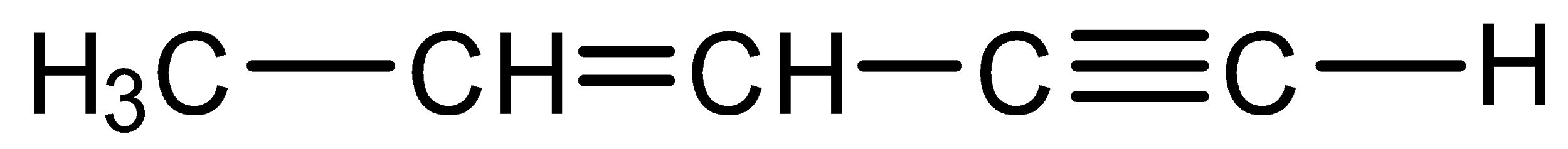 is pent-3-en-1-yne and not pent-2-en-4-yne
is pent-3-en-1-yne and not pent-2-en-4-yne
Reason: Lowest locant rule for multiple bonds is preferred.
a) Both assertion and reason are correct and reason is the correct explanation for assertion.
B) Both assertion and reason are correct but reason is not the correct explanation for assertion.
C) Assertion is correct but reason is incorrect.
D) Assertion is incorrect but reason is correct.
Answer
566.7k+ views
Hint: The answer here is based on the basic concept of organic chemistry that deals with the naming of a compound that is hydrocarbons on the basis if IUPAC rules and the rules which are given by IUPAC gives the correct answer.
Complete Solution :
In the very lower class of organic chemistry, we have studied the basic concepts for naming the compounds which are given by the international union of pure and applied chemistry.
- Let us know to refresh the rules which are required for the above given question so that we can approach the correct answer of whether the assertion and reason are true.
- According to this rule, the double bonds are given the suffix – ene for the molecules and for the triple bond containing molecules, the suffix –yne is used.
- Here, since the given molecule has both the double bond and triple bond in the same molecule, the naming of such types of compounds according to IUPAC rules is based on the lowest locant number.
- This means that we have to choose the longest possible chain of carbon atoms first, whether or not it contains multiple bonds and then choose the chain with more multiple bonds.
Thus, -yne comes first but according to the lowest locant rule it is placed after –ene.
Therefore, both assertion and reason are correct and also reason is the correct explanation for assertion.
So, the correct answer is “Option A”.
Note: Note that if a molecule contains single atoms like chlorine or bromine then the priority is given for that atom which has higher atomic number and that in the functional groups, the highest priority is for the carboxylic acid group which is followed by their derivatives.
Complete Solution :
In the very lower class of organic chemistry, we have studied the basic concepts for naming the compounds which are given by the international union of pure and applied chemistry.
- Let us know to refresh the rules which are required for the above given question so that we can approach the correct answer of whether the assertion and reason are true.
- According to this rule, the double bonds are given the suffix – ene for the molecules and for the triple bond containing molecules, the suffix –yne is used.
- Here, since the given molecule has both the double bond and triple bond in the same molecule, the naming of such types of compounds according to IUPAC rules is based on the lowest locant number.
- This means that we have to choose the longest possible chain of carbon atoms first, whether or not it contains multiple bonds and then choose the chain with more multiple bonds.
Thus, -yne comes first but according to the lowest locant rule it is placed after –ene.
Therefore, both assertion and reason are correct and also reason is the correct explanation for assertion.
So, the correct answer is “Option A”.
Note: Note that if a molecule contains single atoms like chlorine or bromine then the priority is given for that atom which has higher atomic number and that in the functional groups, the highest priority is for the carboxylic acid group which is followed by their derivatives.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




