
Assertion: Decolourisation of \[KMn{O_4}\] solution is used as a test for unsaturation.
Reason: Alkenes on reaction with cold, dilute aqueous solution of potassium permanganate produce vicinal glycols.
A. Both assertion and reason are correct and reason is the correct explanation for Assertion.
B. Both assertion and reason are correct but reason is not the correct explanation for Assertion.
C. Assertion is correct but Reason is incorrect.
D. Both Assertion and Reason are incorrect.
Answer
584.7k+ views
Hint: The potassium permanganate test is also called the Baeyer’s test. It is a test for unsaturation for determining the pressure of double or triple bonds in organic compounds.
Complete step by step answer:
Assertion: Decolourisation of
The solution is used as a test for unsaturation.
Reason: Alkenes on reaction with cold, dilute aqueous solution of potassium permanganate produce vicinal glycols.
First of all, let us try to analyse the assertion given in the question. Acidified, cold, and dilute potassium permanganate is also called Baeyer’s reagent. It is used for determining the presence of carbon-carbon double bonded compounds, called alkenes or carbon-carbon triple bonded compounds called alkynes. And the confirmation of the test is seen when the purple colour of potassium permanganate changes to colourless.
The reaction involved is as follows:
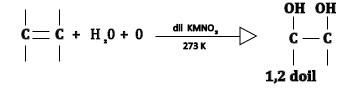
Permanganate (VII) ions in the potassium permanganate are a strong oxidising agent, and in the first instance oxidizes alkene to alkane 1,2-diol. But under acidic condition, the permanganate (VII) ions are reduced to manganese (II) ions and the reaction can be written as:
\[C{H_2} = C{H_2}+ 2{H_2}O + 2Mn{O_4}^ - + 6{H^ + }{\text{ }}\,\,\, \to \,\,\,\,5C{H_2}OH - C{H_2}OH + 2M{n^ + }\]
Alkane 1,2-diols are also called vicinal diols so if we analyse the reason, it seems to be correct and also explains the assertion.
Hence, option (A) is the correct option.
Note:
Students should note that only acidified potassium permanganate gives the unsaturation test. If the physical condition of the \[KMn{O_4}\]is altered, i.e., if it is used in an alkaline medium, then the whole dimension of the reaction changes. In alkaline condition the purple colour permanganate (VII) first changes to green manganate (VII) ions and then further to dark brown manganese (IV) oxide.
Complete step by step answer:
Assertion: Decolourisation of
The solution is used as a test for unsaturation.
Reason: Alkenes on reaction with cold, dilute aqueous solution of potassium permanganate produce vicinal glycols.
First of all, let us try to analyse the assertion given in the question. Acidified, cold, and dilute potassium permanganate is also called Baeyer’s reagent. It is used for determining the presence of carbon-carbon double bonded compounds, called alkenes or carbon-carbon triple bonded compounds called alkynes. And the confirmation of the test is seen when the purple colour of potassium permanganate changes to colourless.
The reaction involved is as follows:

Permanganate (VII) ions in the potassium permanganate are a strong oxidising agent, and in the first instance oxidizes alkene to alkane 1,2-diol. But under acidic condition, the permanganate (VII) ions are reduced to manganese (II) ions and the reaction can be written as:
\[C{H_2} = C{H_2}+ 2{H_2}O + 2Mn{O_4}^ - + 6{H^ + }{\text{ }}\,\,\, \to \,\,\,\,5C{H_2}OH - C{H_2}OH + 2M{n^ + }\]
Alkane 1,2-diols are also called vicinal diols so if we analyse the reason, it seems to be correct and also explains the assertion.
Hence, option (A) is the correct option.
Note:
Students should note that only acidified potassium permanganate gives the unsaturation test. If the physical condition of the \[KMn{O_4}\]is altered, i.e., if it is used in an alkaline medium, then the whole dimension of the reaction changes. In alkaline condition the purple colour permanganate (VII) first changes to green manganate (VII) ions and then further to dark brown manganese (IV) oxide.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




