
Arrange all the isomers of cresol in the increasing order of steric hindrance.
A. Para, meta, ortho
B. Ortho, meta, para
C. Para, ortho, meta
D. Meta, ortho, para
Answer
576.3k+ views
Hint: Steric hindrance is nothing but the stopping capacity of any molecule by other molecules when they are coming to react. If steric hindrance is very high then the chemicals are inert and they won’t react with others due to the presence of bulky groups in it.
Complete step by step answer:
- In the question it is asked to arrange the isomers of cresol in the increasing order of steric hindrance.
- The structure of cresol contains a methyl group in the phenol molecule.
- If the methyl is adjacent (Carbon-2) to the alcohol group in the phenol then it is called ortho cresol and structure of ortho cresol is as follows.
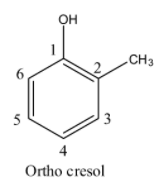
- If the methyl is at carbon-3 in the phenol then it is called meta cresol and structure of meta cresol is as follows.
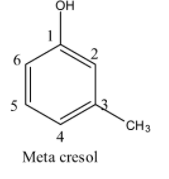
- If the methyl is at carbon-4 in the phenol then it is called para cresol and structure of para cresol is as follows.
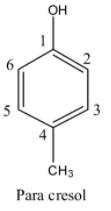
- By observing the structures we can easily say that para cresol has less steric hindrance due to the presence of substituents or bulky groups at so far distance to each other.
- Meta cresol has somewhat steric hindrance when compared to para cresol.
- Coming to ortho cresol, it has high steric hindrance due to the presence of bulky groups adjacent to each other.
- Therefore increasing order of steric hindrance in isomers of cresol is as follows.
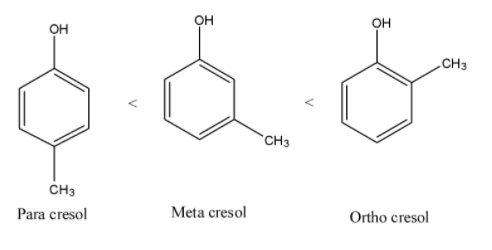
Note: Due to the presence of large steric hindrance in ortho cresol it won’t react that much easily with other molecules. Among the isomers of cresol the ortho cresol has less reactivity towards other molecules.
Complete step by step answer:
- In the question it is asked to arrange the isomers of cresol in the increasing order of steric hindrance.
- The structure of cresol contains a methyl group in the phenol molecule.
- If the methyl is adjacent (Carbon-2) to the alcohol group in the phenol then it is called ortho cresol and structure of ortho cresol is as follows.

- If the methyl is at carbon-3 in the phenol then it is called meta cresol and structure of meta cresol is as follows.

- If the methyl is at carbon-4 in the phenol then it is called para cresol and structure of para cresol is as follows.

- By observing the structures we can easily say that para cresol has less steric hindrance due to the presence of substituents or bulky groups at so far distance to each other.
- Meta cresol has somewhat steric hindrance when compared to para cresol.
- Coming to ortho cresol, it has high steric hindrance due to the presence of bulky groups adjacent to each other.
- Therefore increasing order of steric hindrance in isomers of cresol is as follows.

Note: Due to the presence of large steric hindrance in ortho cresol it won’t react that much easily with other molecules. Among the isomers of cresol the ortho cresol has less reactivity towards other molecules.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




