
What are the postulates of Bohr's model of an atom? Show diagrammatically the electron distribution in a sodium atom and a sodium ion and also give their atomic number.
Answer
517.2k+ views
Hint :
We must note that Neil Bohr presented the Bohr model of the atom in 1915. With the alteration of Rutherford's atomic model, it became a possibility. According to Rutherford's model, a positively charged nucleus is surrounded by negatively charged electrons. Bohr changed the atomic structure model by stating that electrons are confined to fixed orbitals known as shells. Each orbit, he explained, has a set energy level. Rutherford identified an atom's nucleus, which Bohr modified into electrons and their energy levels.
Complete Step By Step Answer:
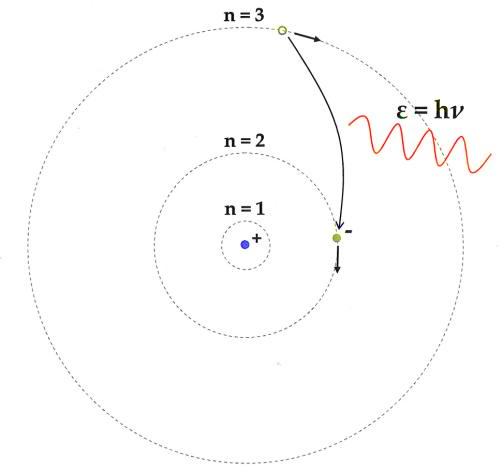
The following are the postulates of Bohr's atomic model:
1. Negatively charged electrons in an atom rotate around the positively charged nucleus in a spiral direction known as orbits or shells.
2. The circular orbits are known as orbital shells because each orbit or shell has a set energy.
3. The quantum number is an integer n =1,2,3,... that denotes the energy levels. This collection of quantum numbers begins on the nucleus's side, with n=1 being the lowest energy density. The orbits n=1,2,3,4 are known as K, L, M, N.... shells, and an electron is assumed to be in the ground state when it approaches the lowest energy level.
4. Electrons in an atom gain energy as they move from a lower energy level to a higher energy level, and they lose energy when they go from a higher energy level to a lower energy level.
self made
self made
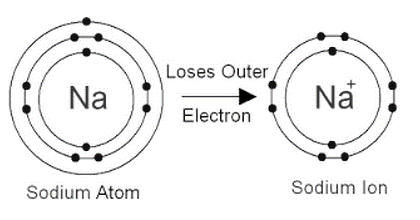
Sodium's atomic number is 11.
An element's atomic number (Z) is proportional to the number of protons in the atom. The sodium atom has 11 protons due to its atomic number of 11. Since the atom is electrically neutral, the number of electrons equals the number of protons. The elimination of one electron from a sodium atom produces a positively charged sodium ion (Na⁺). A sodium ion, thus, has 11 – 1 = 10 electrons. As a result, the sodium ion's electrical distribution would be 2,8. Since the sodium atom and the sodium ion each have 11 protons, the atomic number of the sodium atom and the sodium ion is the same.
Note :
To become a sodium ion, the sodium atom loses its outer electron. There are already 11 protons in the sodium ion (11 positive charges). As a result, the atomic number of sodium ion is also 11, but there are just 10 electrons now (10 negative charges). The + symbol indicates that the sodium ion has an additional positive charge.
We must note that Neil Bohr presented the Bohr model of the atom in 1915. With the alteration of Rutherford's atomic model, it became a possibility. According to Rutherford's model, a positively charged nucleus is surrounded by negatively charged electrons. Bohr changed the atomic structure model by stating that electrons are confined to fixed orbitals known as shells. Each orbit, he explained, has a set energy level. Rutherford identified an atom's nucleus, which Bohr modified into electrons and their energy levels.
Complete Step By Step Answer:
The following are the postulates of Bohr's atomic model:
1. Negatively charged electrons in an atom rotate around the positively charged nucleus in a spiral direction known as orbits or shells.
2. The circular orbits are known as orbital shells because each orbit or shell has a set energy.
3. The quantum number is an integer n =1,2,3,... that denotes the energy levels. This collection of quantum numbers begins on the nucleus's side, with n=1 being the lowest energy density. The orbits n=1,2,3,4 are known as K, L, M, N.... shells, and an electron is assumed to be in the ground state when it approaches the lowest energy level.
4. Electrons in an atom gain energy as they move from a lower energy level to a higher energy level, and they lose energy when they go from a higher energy level to a lower energy level.
self made
self made


Sodium's atomic number is 11.
An element's atomic number (Z) is proportional to the number of protons in the atom. The sodium atom has 11 protons due to its atomic number of 11. Since the atom is electrically neutral, the number of electrons equals the number of protons. The elimination of one electron from a sodium atom produces a positively charged sodium ion (Na⁺). A sodium ion, thus, has 11 – 1 = 10 electrons. As a result, the sodium ion's electrical distribution would be 2,8. Since the sodium atom and the sodium ion each have 11 protons, the atomic number of the sodium atom and the sodium ion is the same.
Note :
To become a sodium ion, the sodium atom loses its outer electron. There are already 11 protons in the sodium ion (11 positive charges). As a result, the atomic number of sodium ion is also 11, but there are just 10 electrons now (10 negative charges). The + symbol indicates that the sodium ion has an additional positive charge.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




