
How are the following conversions carried out?
1.Chlorobenzene to benzoic acid
2.Chlorobenzene to benzene
Answer
573.3k+ views
Hint: We have to know that chlorobenzene is a haloarene. It is an aromatic substituted compound that contains a chloro group attached to a benzene ring. We can obtain Benzoic acid from chlorobenzene by treating sodium and methyl chloride in the presence of dry ether with chlorobenzene, and the product is formed. The formed product is then oxidized to form benzoic acid. The benzoic acid is then reacted with sodium hydroxide and calcium oxide to form benzene.
Complete answer:
1.Formation of benzoic acid from chlorobenzene:
We know that chlorobenzene is a haloarenes (aryl halides) that contains a chloro group linked to benzene rings. We can obtain benzoic acid from chlorobenzene when we treat chlorobenzene with sodium and methyl chloride in the presence of ether to form toluene. Toluene undergoes oxidation using oxidizing agents such as potassium permanganate, and sulfuric acid to form benzoic acid. We can write the chemical equation as,
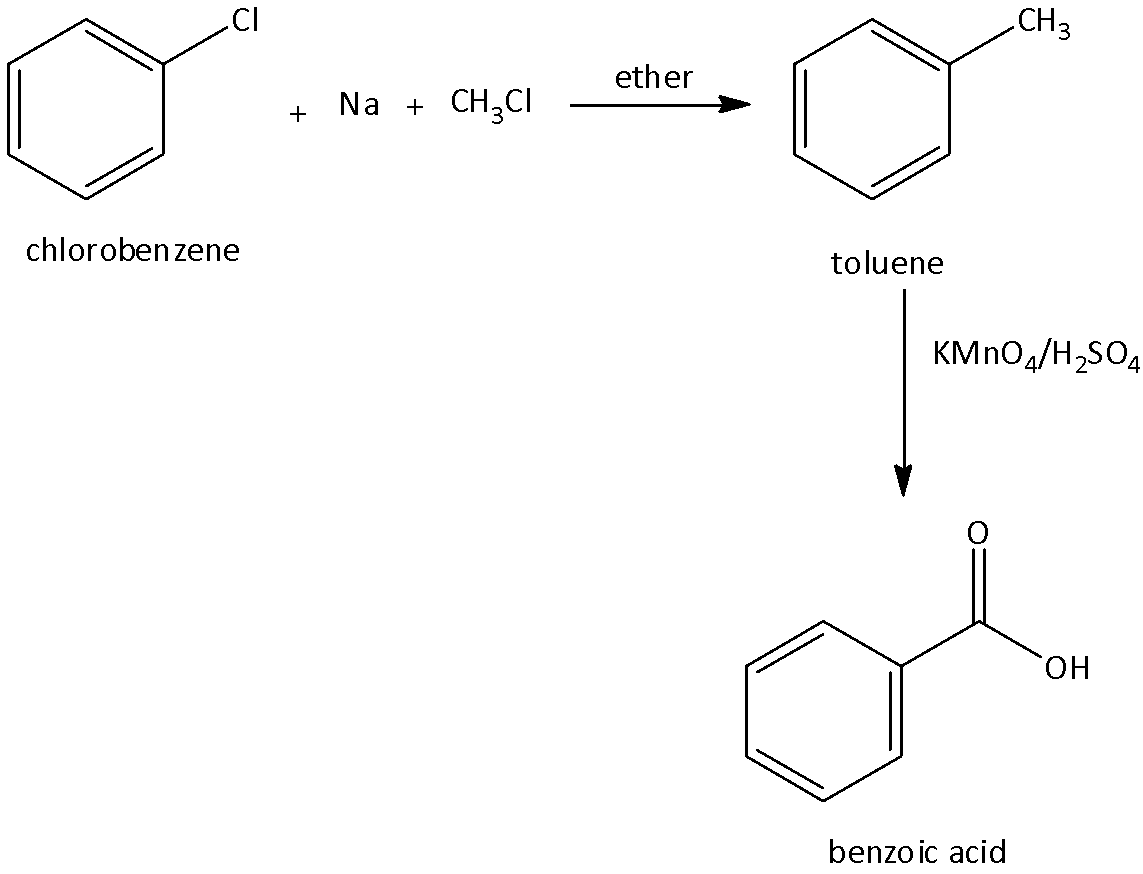
2.Formation of benzene from chlorobenzene:
We know that chlorobenzene is a haloarenes (aryl halides) that contains a chloro group linked to benzene rings. We can obtain benzene from chlorobenzene when we treat chlorobenzene with sodium and methyl chloride in the presence of ether to form toluene. Toluene undergoes oxidation using oxidizing agents such as potassium permanganate, and sulfuric acid to form benzoic acid. Benzoic acid is then treated with sodium to form sodium phenoxide. The formed sodium phenoxide is then treated with sodium hydroxide and calcium oxide to form benzene. We can write the chemical reaction as,
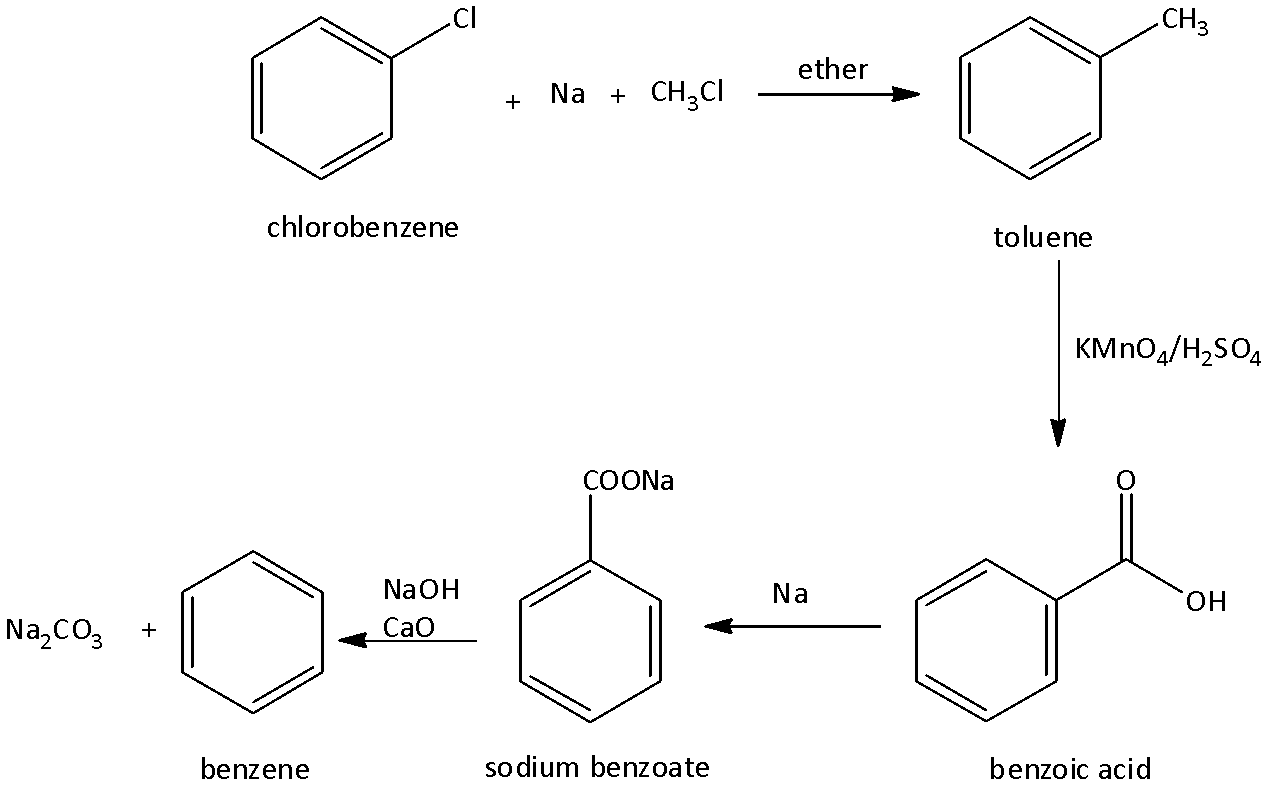
Note:
We have to remember that the chlorobenzene could be converted to benzoic acid using Grignard reagent and also using iodide-silica gel catalyst. The carboxylation of chlorobenzene with carbon monoxide and water at high pressure in the presence of iodide-silica gel catalyst gives benzoic acid as the product. We can write the chemical reaction as,
${C_6}{H_5}Cl + CO + {H_2}O \to {C_6}{H_5}COOH + HCl$
We can also prepare benzene from chlorobenzene, by treating chlorobenzene with hydrogen in the presence of sodium hydroxide/Ni-Al alloy. We can write the chemical reaction as,
${C_6}{H_5}Cl + 2\left[ H \right]\underset{{NaOH}}{\overset{{Ni - Al\,Alloy}}{\longleftrightarrow}}{C_6}{H_6} + HCl$
We can also convert chlorobenzene to sodium phenoxide, convert sodium phenoxide to phenol, then convert phenol to benzene using zinc dust.
Complete answer:
1.Formation of benzoic acid from chlorobenzene:
We know that chlorobenzene is a haloarenes (aryl halides) that contains a chloro group linked to benzene rings. We can obtain benzoic acid from chlorobenzene when we treat chlorobenzene with sodium and methyl chloride in the presence of ether to form toluene. Toluene undergoes oxidation using oxidizing agents such as potassium permanganate, and sulfuric acid to form benzoic acid. We can write the chemical equation as,

2.Formation of benzene from chlorobenzene:
We know that chlorobenzene is a haloarenes (aryl halides) that contains a chloro group linked to benzene rings. We can obtain benzene from chlorobenzene when we treat chlorobenzene with sodium and methyl chloride in the presence of ether to form toluene. Toluene undergoes oxidation using oxidizing agents such as potassium permanganate, and sulfuric acid to form benzoic acid. Benzoic acid is then treated with sodium to form sodium phenoxide. The formed sodium phenoxide is then treated with sodium hydroxide and calcium oxide to form benzene. We can write the chemical reaction as,

Note:
We have to remember that the chlorobenzene could be converted to benzoic acid using Grignard reagent and also using iodide-silica gel catalyst. The carboxylation of chlorobenzene with carbon monoxide and water at high pressure in the presence of iodide-silica gel catalyst gives benzoic acid as the product. We can write the chemical reaction as,
${C_6}{H_5}Cl + CO + {H_2}O \to {C_6}{H_5}COOH + HCl$
We can also prepare benzene from chlorobenzene, by treating chlorobenzene with hydrogen in the presence of sodium hydroxide/Ni-Al alloy. We can write the chemical reaction as,
${C_6}{H_5}Cl + 2\left[ H \right]\underset{{NaOH}}{\overset{{Ni - Al\,Alloy}}{\longleftrightarrow}}{C_6}{H_6} + HCl$
We can also convert chlorobenzene to sodium phenoxide, convert sodium phenoxide to phenol, then convert phenol to benzene using zinc dust.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




