
How are the following conversions carried out?
Propene \[\to \]Propan-2-ol
Answer
596.4k+ views
Hint: In the given reaction an alkene is going to convert into alcohol without changing its number of carbons in the reactant. The reaction is only possible by using sulphuric acid as a reagent.
Complete step by step solution:
1) The conversion of propene to propan-2-ol is a two-step process.
The complete reaction is as follows.
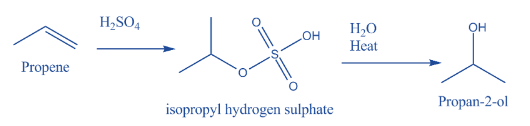
2) The above reaction is a two-step reaction.
- In the first step sulphuric acid reacts with propene and forms an intermediate compound. The name of the intermediate compound is isopropyl hydrogen sulphate.
- In the second step the formed isopropyl hydrogen sulphate converted into propan-2-ol by the presence of water and heat.
> It is a type of additional reaction because alkene is converted into saturated compounds (alcohol).
> The formation of the product is easily confirmed by the sweet smell.
Alcohols with small chains given sweet smell during their synthesis.
So, the conversion of propene to propan-2-ol is possible by the hydration of propene in presence of sulphuric acid.
> Propan-2-ol is also called Isopropyl alcohol.
> Isopropyl alcohol is a colorless, flammable chemical compound with a strong odor.
> In the isopropyl alcohol hydroxyl group linked to second carbon, it is the simplest example of a secondary alcohol.
Note: Don’t be confused with the words alkene and alcohol.
Alkene contains double bonds in its structure.
Alcohol contains an \[-OH\] functional group in its structure.
Complete step by step solution:
1) The conversion of propene to propan-2-ol is a two-step process.
The complete reaction is as follows.

2) The above reaction is a two-step reaction.
- In the first step sulphuric acid reacts with propene and forms an intermediate compound. The name of the intermediate compound is isopropyl hydrogen sulphate.
- In the second step the formed isopropyl hydrogen sulphate converted into propan-2-ol by the presence of water and heat.
> It is a type of additional reaction because alkene is converted into saturated compounds (alcohol).
> The formation of the product is easily confirmed by the sweet smell.
Alcohols with small chains given sweet smell during their synthesis.
So, the conversion of propene to propan-2-ol is possible by the hydration of propene in presence of sulphuric acid.
> Propan-2-ol is also called Isopropyl alcohol.
> Isopropyl alcohol is a colorless, flammable chemical compound with a strong odor.
> In the isopropyl alcohol hydroxyl group linked to second carbon, it is the simplest example of a secondary alcohol.
Note: Don’t be confused with the words alkene and alcohol.
Alkene contains double bonds in its structure.
Alcohol contains an \[-OH\] functional group in its structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




