
What are the excited states and the hybridization of \[As{O_4}^{3 - }\] and \[P{F_4}^ - \] ?
Answer
468.9k+ views
Hint: Quantum mechanics is an important branch of science. It deals with the energy’s states and excitations. The quantum state in a molecule that has higher energy than the ground state can be known as the excited state. There will be an infinite number of excited states of a molecule.
Complete answer:
Given molecules are \[As{O_4}^{3 - }\] and \[P{F_4}^ - \]
These both molecules have an infinite number of excited states.
In \[As{O_4}^{3 - }\] , the central metal atom is arsenic. Arsenic has the atomic number of \[33\] , the valence electrons in arsenic are five. Whereas oxygen has an atomic number of \[8\] , it has \[6\] valence electrons. as there were four oxygen atoms in \[As{O_4}^{3 - }\] , the total number of valence electrons will be equal to \[24\] and there is a negative charge of \[ - 3\] . thus, the total number of electrons in \[As{O_4}^{3 - }\] will be equal to \[35\]
These \[35\] electrons are arranged as follows:
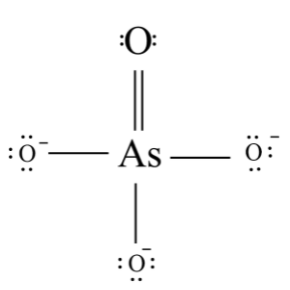
This molecule has steric number \[4\] , and also has \[A{X_4}\] system which has a geometry of tetrahedral and has hybridization of \[s{p^3}\]
In \[P{F_4}^ - \] , the central metal atom is phosphorous, with five valence electrons and each fluorine has \[7\] valence electrons as four fluorine atoms are there, the total valence electrons of four fluorine atoms will be \[28\] and this molecule has one negative charge. Thus, the total valence electrons will be equal to \[34\] .
These \[34\] electrons are arranged as follows:
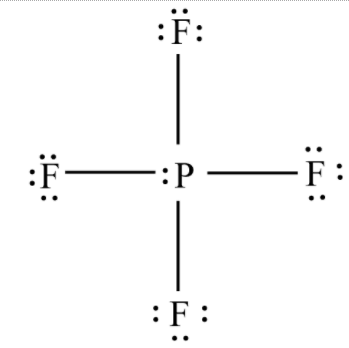
This molecule has a steric number of \[5\] and has the hybridization of \[s{p^3}d\] with \[A{X_4}E\] system.
Note: The steric number can be given by the sum of the bonding pair of electrons and lone pair of electrons on the central metal atoms but not on terminal atoms. The atom with low electronegativity occupies the central position of a molecule.
Complete answer:
Given molecules are \[As{O_4}^{3 - }\] and \[P{F_4}^ - \]
These both molecules have an infinite number of excited states.
In \[As{O_4}^{3 - }\] , the central metal atom is arsenic. Arsenic has the atomic number of \[33\] , the valence electrons in arsenic are five. Whereas oxygen has an atomic number of \[8\] , it has \[6\] valence electrons. as there were four oxygen atoms in \[As{O_4}^{3 - }\] , the total number of valence electrons will be equal to \[24\] and there is a negative charge of \[ - 3\] . thus, the total number of electrons in \[As{O_4}^{3 - }\] will be equal to \[35\]
These \[35\] electrons are arranged as follows:

This molecule has steric number \[4\] , and also has \[A{X_4}\] system which has a geometry of tetrahedral and has hybridization of \[s{p^3}\]
In \[P{F_4}^ - \] , the central metal atom is phosphorous, with five valence electrons and each fluorine has \[7\] valence electrons as four fluorine atoms are there, the total valence electrons of four fluorine atoms will be \[28\] and this molecule has one negative charge. Thus, the total valence electrons will be equal to \[34\] .
These \[34\] electrons are arranged as follows:

This molecule has a steric number of \[5\] and has the hybridization of \[s{p^3}d\] with \[A{X_4}E\] system.
Note: The steric number can be given by the sum of the bonding pair of electrons and lone pair of electrons on the central metal atoms but not on terminal atoms. The atom with low electronegativity occupies the central position of a molecule.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Which Country is Called "The Land of Festivals"?

What type of cell is found in the Seminiferous tub class 10 biology CBSE

What are the public facilities provided by the government? Also explain each facility




