
What are induced dipole-dipole forces?
Answer
521.4k+ views
Hint: In chemical reactions, only one major force is at work: the electromagnetic force. Gravity, electromagnetic force, strong nuclear force, and weak nuclear force are the four basic forces of the universe.
Complete step by step answer:
Attraction and repulsion between molecules, atoms, and surfaces, as well as other intermolecular forces, are all examples of Van der Waals forces. Dispersion, dipole-dipole, and hydrogen are the three forms of van der Waals forces. These forces exist between molecules or atoms that are not polar. The electron cloud will be uniformly distributed between the two atoms since the molecules are nonpolar.
The electron cloud does not remain static. It travels around the atom at a rapid pace. As a result, the cloud may be more concentrated on one side of the molecule than the other at any given time. As a result, the side with the highest concentration of electrons will become $ {\delta ^ + } $ , while the side with the lowest concentration will become $ {\delta ^ - } $ this is known as a temporary dipole moment. Since they are separated by such a small distance, these charges are just minor.
The diagram below will help to clarify things.
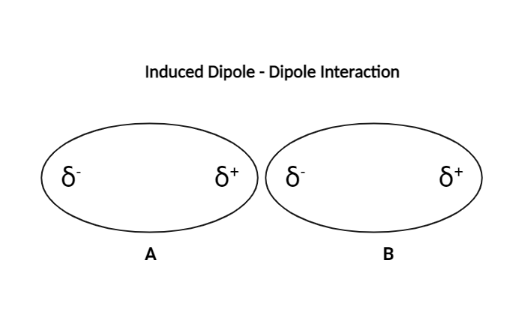
A molecule is represented by each oval. As the $ {\delta ^ + } $ end of molecule A approaches molecules B, the positive charge induces a negative charge in the B. Remember that opposite charges attract, so $ {\delta ^ + } $ of molecule A would make B molecule’s left side negative. As a result, molecule A will attract the electron cloud in B to the left, allowing the right side of molecule B to become slightly positive. As a result, a weak force of attraction forms between the two molecules. The same idea applies to the $ {\delta ^ - } $ end of the molecule.
So, we can say that induced dipole-dipole force is simply another term for Van der Waals forces.
Note:
It is important to remember that the induced dipole-dipole attraction is a weak one. This attraction occurs when a polar molecule causes a dipole in an atom or a nonpolar molecule by disrupting the nonpolar species' electron arrangement.
Complete step by step answer:
Attraction and repulsion between molecules, atoms, and surfaces, as well as other intermolecular forces, are all examples of Van der Waals forces. Dispersion, dipole-dipole, and hydrogen are the three forms of van der Waals forces. These forces exist between molecules or atoms that are not polar. The electron cloud will be uniformly distributed between the two atoms since the molecules are nonpolar.
The electron cloud does not remain static. It travels around the atom at a rapid pace. As a result, the cloud may be more concentrated on one side of the molecule than the other at any given time. As a result, the side with the highest concentration of electrons will become $ {\delta ^ + } $ , while the side with the lowest concentration will become $ {\delta ^ - } $ this is known as a temporary dipole moment. Since they are separated by such a small distance, these charges are just minor.
The diagram below will help to clarify things.

A molecule is represented by each oval. As the $ {\delta ^ + } $ end of molecule A approaches molecules B, the positive charge induces a negative charge in the B. Remember that opposite charges attract, so $ {\delta ^ + } $ of molecule A would make B molecule’s left side negative. As a result, molecule A will attract the electron cloud in B to the left, allowing the right side of molecule B to become slightly positive. As a result, a weak force of attraction forms between the two molecules. The same idea applies to the $ {\delta ^ - } $ end of the molecule.
So, we can say that induced dipole-dipole force is simply another term for Van der Waals forces.
Note:
It is important to remember that the induced dipole-dipole attraction is a weak one. This attraction occurs when a polar molecule causes a dipole in an atom or a nonpolar molecule by disrupting the nonpolar species' electron arrangement.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




