
Why are antibonding orbitals higher in energy?
Answer
507.3k+ views
Hint : The molecular orbital theory is based on chemical bonding which is used to describe the structures and properties of different molecules. It states that each atom tends to combine together to form molecular orbitals and this theory also explains that the molecular orbitals are basically linear combinations of atomic orbitals.
Complete Step By Step Answer:
The important points which cover the key features of molecular orbital theory are as follows:
1. The total number of molecular orbitals formed are always equal to the total number of atomic orbitals participating in bonding.
2. There exists three types of molecular orbitals which are bonding molecular orbital, antibonding molecular orbital and antibonding molecular orbitals.
3. The electrons are filled into molecular orbitals in increasing order i.e., from the orbital with lowest energy to the orbital with highest energy.
4. For the formation of molecular orbitals, the most effective combinations of atomic orbitals occur when the atomic orbitals which are combining comprise similar energies.
Now, let’s discuss types of molecular orbitals.
Bonding molecular orbitals: These are formed when constructive overlapping of atomic orbitals takes place and probability of finding electrons is higher in case of bonding molecular orbitals. In these orbitals, the electron density in the internuclear distance is high due to which the nuclei are shielded from each other and thus experience less repulsions. These are represented by $ \sigma ,\;\pi ,\;\delta $ . A sketch of bonding molecular orbitals is given below:
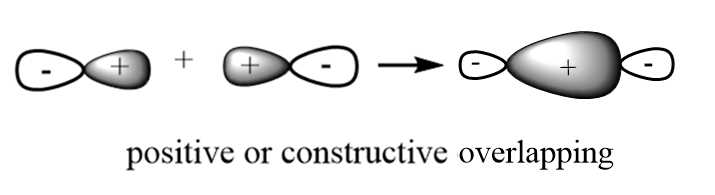
Anti-Bonding molecular orbitals: These are formed when destructive overlapping of atomic orbitals takes place and probability of finding electrons is very low in case of anti-bonding molecular orbitals. In these orbitals, the electron density in the internuclear distance is low due to which the nuclei are directly exposed to each other and thus experience high repulsive forces. These are represented by $ {\sigma ^*},\;{\pi ^*},\;{\delta ^*} $ . A sketch of anti-bonding molecular orbitals is given below:
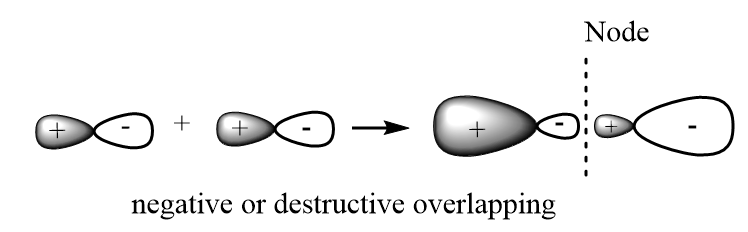
Non-bonding molecular orbitals: In this case, due to the lack of symmetry in the compatibility of two atomic orbitals which are participating in bond formation, the molecular orbitals have no positive or negative interactions with each other and thus, do not affect the bond formed between the two atoms.
From the above points, it is clear that the electrons in the bonding molecular orbitals are attracted by the nuclei whereas in case of anti-bonding molecular orbitals, the nuclei repel each other. Thus, we can conclude that atomic orbitals are always higher in energy.
Note :
It is important to note that the valence bond theory failed to explain the formation of multiple bonds in the molecule whose bond order lies between a single and a double bond like the bonds in resonance stabilized molecules. Since, MOT reflects the geometries of the molecules so it was proved to be more useful than the valence bond theory in this aspect.
Complete Step By Step Answer:
The important points which cover the key features of molecular orbital theory are as follows:
1. The total number of molecular orbitals formed are always equal to the total number of atomic orbitals participating in bonding.
2. There exists three types of molecular orbitals which are bonding molecular orbital, antibonding molecular orbital and antibonding molecular orbitals.
3. The electrons are filled into molecular orbitals in increasing order i.e., from the orbital with lowest energy to the orbital with highest energy.
4. For the formation of molecular orbitals, the most effective combinations of atomic orbitals occur when the atomic orbitals which are combining comprise similar energies.
Now, let’s discuss types of molecular orbitals.
Bonding molecular orbitals: These are formed when constructive overlapping of atomic orbitals takes place and probability of finding electrons is higher in case of bonding molecular orbitals. In these orbitals, the electron density in the internuclear distance is high due to which the nuclei are shielded from each other and thus experience less repulsions. These are represented by $ \sigma ,\;\pi ,\;\delta $ . A sketch of bonding molecular orbitals is given below:

Anti-Bonding molecular orbitals: These are formed when destructive overlapping of atomic orbitals takes place and probability of finding electrons is very low in case of anti-bonding molecular orbitals. In these orbitals, the electron density in the internuclear distance is low due to which the nuclei are directly exposed to each other and thus experience high repulsive forces. These are represented by $ {\sigma ^*},\;{\pi ^*},\;{\delta ^*} $ . A sketch of anti-bonding molecular orbitals is given below:

Non-bonding molecular orbitals: In this case, due to the lack of symmetry in the compatibility of two atomic orbitals which are participating in bond formation, the molecular orbitals have no positive or negative interactions with each other and thus, do not affect the bond formed between the two atoms.
From the above points, it is clear that the electrons in the bonding molecular orbitals are attracted by the nuclei whereas in case of anti-bonding molecular orbitals, the nuclei repel each other. Thus, we can conclude that atomic orbitals are always higher in energy.
Note :
It is important to note that the valence bond theory failed to explain the formation of multiple bonds in the molecule whose bond order lies between a single and a double bond like the bonds in resonance stabilized molecules. Since, MOT reflects the geometries of the molecules so it was proved to be more useful than the valence bond theory in this aspect.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




