
Aniline on reaction with con. ${{H}_{2}}S{{O}_{4}}$ gives X. If X is heated, the product is:
A. Sulphanilic acid
B. Sulphonamide
C. Benzene sulphonyl chloride
D. m-amino benzene sulphonic acid.
Answer
531.6k+ views
Hint: Aniline is an organic compound having the molecular formula ${{C}_{6}}{{H}_{5}}N{{H}_{2}}$ it consists of a phenyl group attached with an amino group and aniline is also known as the simplest aromatic compound with various industrial uses.
Complete answer:
Anile has the odor of rotten fish and it ignites easily, burns with a smoky flame which defines its characteristics of aromatic compounds. Chemically aniline is considered as electron rich benzene derivative which fastly reacts in electrophilic aromatic substitution reactions.
Now the reaction of aniline with conc. can be defined by the following mechanism:
In the first step aniline gets heated with concentrated ${{H}_{2}}S{{O}_{4}}$and form the compound which after losing water molecule form zwitterion which can be known by the name inner salt. It can be defined as a molecule which contains an equal number of positively and negatively charged functional groups. In case of zwitterion positive charge is placed on a quaternary ammonium group.
After the formation of zwitterion rearrangement of protons take place which form the final product known by the name sulphanilic acid. Mechanism is shown as follows:
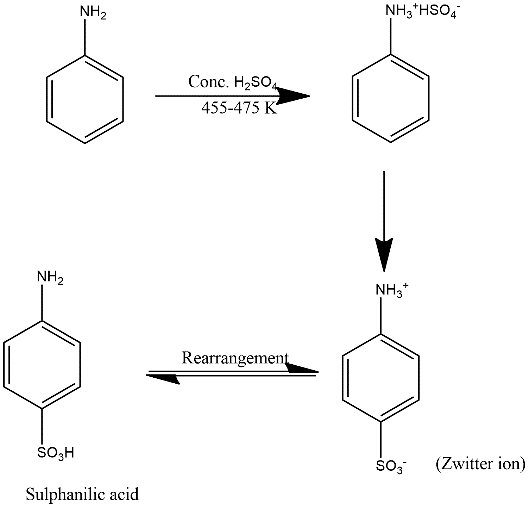
Hence option A is the correct answer.
Note:
There are many applications of aniline, the largest one is preparation of methylenedianiline and related compounds by condensation with formaldehyde. Other uses include rubber processing chemicals like herbicides, dyes and pigments.
Complete answer:
Anile has the odor of rotten fish and it ignites easily, burns with a smoky flame which defines its characteristics of aromatic compounds. Chemically aniline is considered as electron rich benzene derivative which fastly reacts in electrophilic aromatic substitution reactions.
Now the reaction of aniline with conc. can be defined by the following mechanism:
In the first step aniline gets heated with concentrated ${{H}_{2}}S{{O}_{4}}$and form the compound which after losing water molecule form zwitterion which can be known by the name inner salt. It can be defined as a molecule which contains an equal number of positively and negatively charged functional groups. In case of zwitterion positive charge is placed on a quaternary ammonium group.
After the formation of zwitterion rearrangement of protons take place which form the final product known by the name sulphanilic acid. Mechanism is shown as follows:

Hence option A is the correct answer.
Note:
There are many applications of aniline, the largest one is preparation of methylenedianiline and related compounds by condensation with formaldehyde. Other uses include rubber processing chemicals like herbicides, dyes and pigments.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




