
An organic compound with the formula ${C_6}{H_{12}}{O_6}$ forms a yellow crystalline solid with phenylhydrazine and gives a mixture of sorbitol and mannitol when reduced with sodium. Which among the following could be the compound?
A.Fructose
B.Glucose
C.Mannose
D.Sucrose
Answer
570k+ views
Hint:
All the given options are carbohydrates. Fructose is also called as fruit sugar since it is present in fruits. Glucose is also known as dextrose since it occurs in the dextrorotatory form in nature. These are present in two forms- open chain and ring form.
Complete step by step answer:
Glucose is a monosaccharide that is aldohexose sugar. Fructose is also a monosaccharide that is a ketohexose sugar.
Monosaccharides are the single unit carbohydrates which cannot be broken down into lower sugars upon hydrolysis.
Both glucose and fructose have the molecular formula ${C_6}{H_{12}}{O_6}$ .
Sucrose is a disaccharide with the molecular formula ${C_{12}}{H_{22}}{O_{11}}$ . Mannose is also an aldohexose sugar with molecular formula ${C_6}{H_{12}}{O_6}$ .
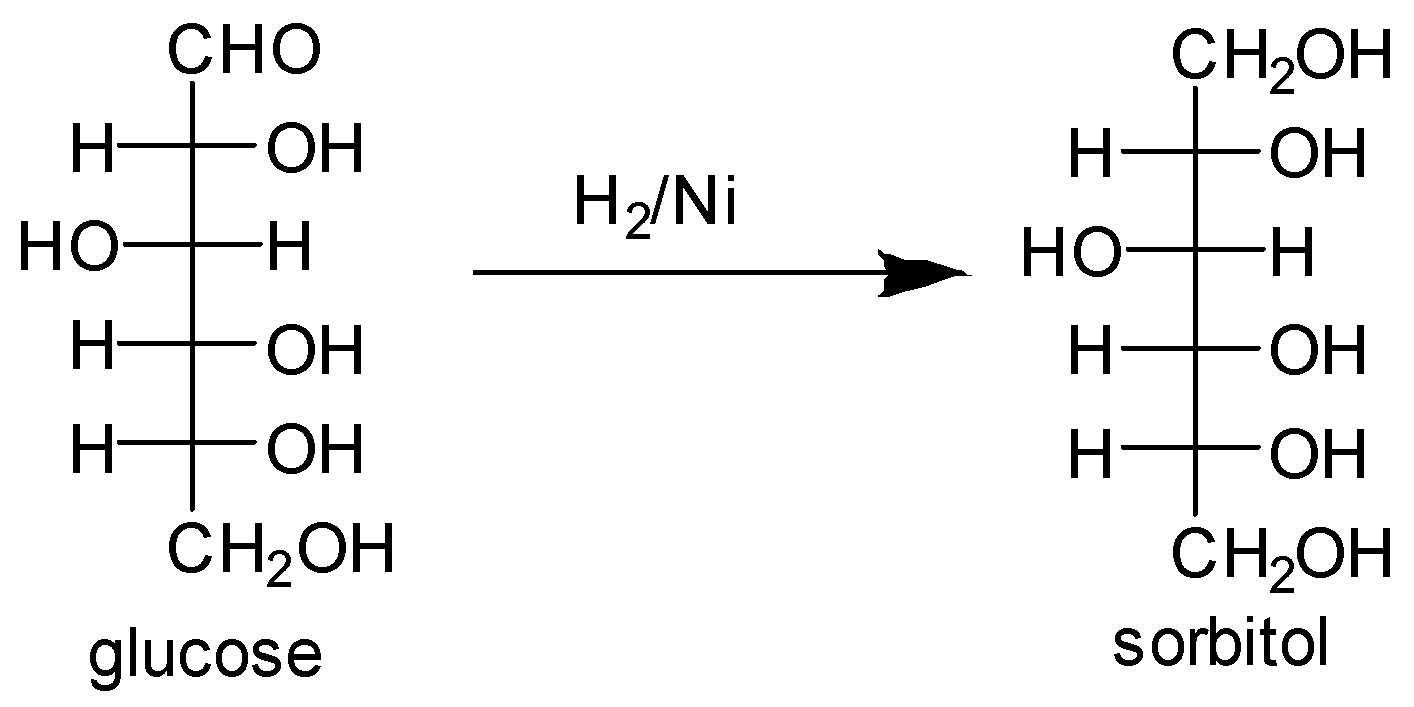
Glucose on reduction with phenylhydrazine forms an osazone derivative that is a yellow solid. Glucose when reduced with sodium amalgam, sorbitol is formed as the major product. Sorbitol is a polyhydroxy alcohol.
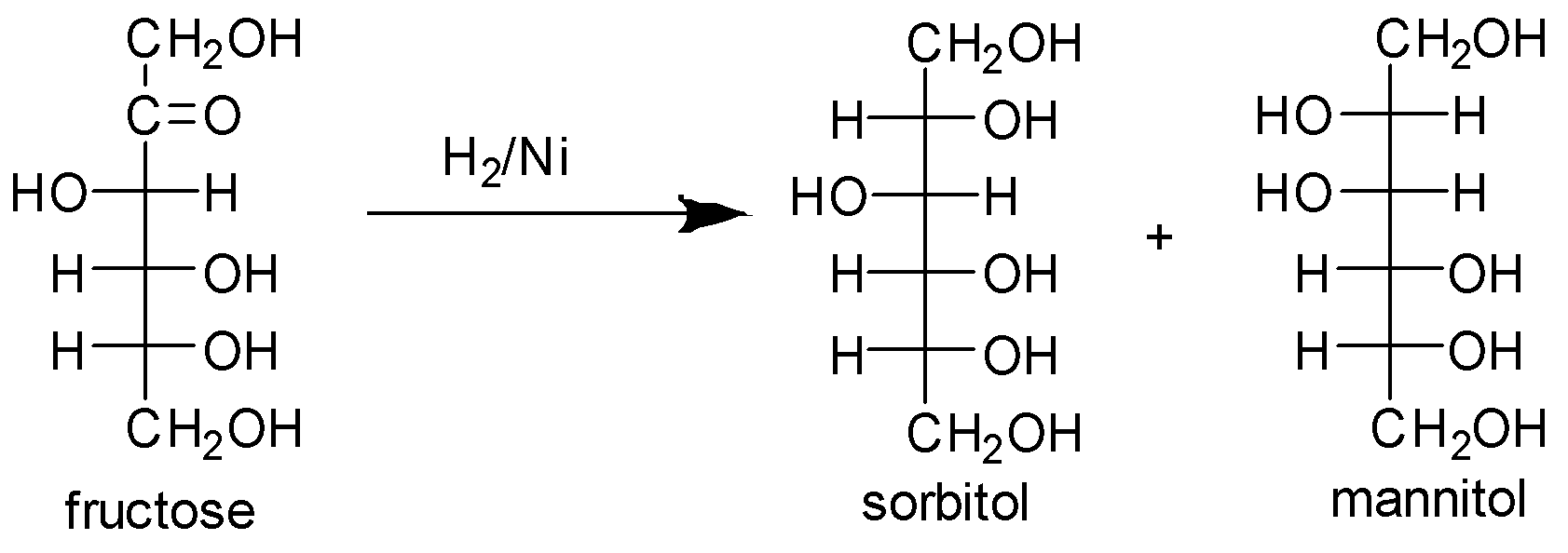
Fructose forms a yellow crystalline solid on reduction with phenylhydrazine. Also fructose on reduction with sodium amalgam gives a mixture of sorbitol and mannitol.
Sucrose is a disaccharide with molecular formula ${C_{12}}{H_{22}}{O_{11}}$ . But the given compound has formula ${C_6}{H_{12}}{O_6}$ . So sucrose is the incorrect option.
Mannose on reduction with sodium does not give a mixture of sorbitol and mannitol. Hence it is also the incorrect option.
From the above reaction we can see that fructose on reduction with sodium gives a mixture of sorbitol and mannitol and forms a yellow solid with phenylhydrazine.
The correct option is A.
Note:On hydrolysis of sucrose, we get a mixture of glucose and fructose. Sucrose is used as a sweetening agent.
-Mannose is a C-2 epimer of glucose. Mannose differ at the configuration at second carbon with respect to structure of glucose.
All the given options are carbohydrates. Fructose is also called as fruit sugar since it is present in fruits. Glucose is also known as dextrose since it occurs in the dextrorotatory form in nature. These are present in two forms- open chain and ring form.
Complete step by step answer:
Glucose is a monosaccharide that is aldohexose sugar. Fructose is also a monosaccharide that is a ketohexose sugar.
Monosaccharides are the single unit carbohydrates which cannot be broken down into lower sugars upon hydrolysis.
Both glucose and fructose have the molecular formula ${C_6}{H_{12}}{O_6}$ .
Sucrose is a disaccharide with the molecular formula ${C_{12}}{H_{22}}{O_{11}}$ . Mannose is also an aldohexose sugar with molecular formula ${C_6}{H_{12}}{O_6}$ .
Glucose on reduction with phenylhydrazine forms an osazone derivative that is a yellow solid. Glucose when reduced with sodium amalgam, sorbitol is formed as the major product. Sorbitol is a polyhydroxy alcohol.

Fructose forms a yellow crystalline solid on reduction with phenylhydrazine. Also fructose on reduction with sodium amalgam gives a mixture of sorbitol and mannitol.

Sucrose is a disaccharide with molecular formula ${C_{12}}{H_{22}}{O_{11}}$ . But the given compound has formula ${C_6}{H_{12}}{O_6}$ . So sucrose is the incorrect option.
Mannose on reduction with sodium does not give a mixture of sorbitol and mannitol. Hence it is also the incorrect option.
From the above reaction we can see that fructose on reduction with sodium gives a mixture of sorbitol and mannitol and forms a yellow solid with phenylhydrazine.
The correct option is A.
Note:On hydrolysis of sucrose, we get a mixture of glucose and fructose. Sucrose is used as a sweetening agent.
-Mannose is a C-2 epimer of glucose. Mannose differ at the configuration at second carbon with respect to structure of glucose.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




