
An acid halide $\left( {\rm{X}} \right)$ reacts with excess of Grignard reagent. The product \[\left( {\rm{Y}} \right)\] can be:
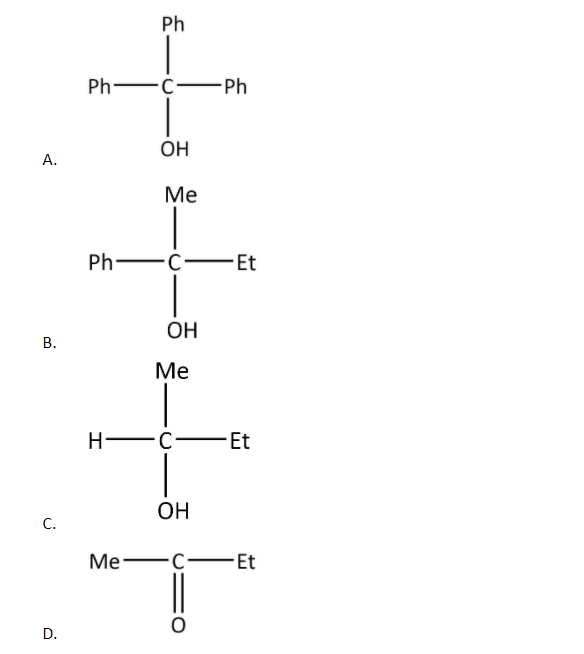

Answer
583.2k+ views
Hint: The product can be deduced by using the understanding of the mechanism of the reaction between an acid halide and Grignard reagent.
Step by step answer: Grignard reagent is an organo-metallic compound with a general formula ${\rm{RMgX}}$ in which an alkyl group $\left( {\rm{R}} \right)$ is attached to magnesium and there is a halide $\left( {\rm{X}} \right)$ present as well. In ${\rm{R}} - {\rm{MgX}}$ bond is covalent but due to Electronegativity difference, it is polar and ${\rm{RMg}} - {\rm{X}}$ bond is ionic in nature. This gives rise to charge separations as ${{\rm{R}}^{\delta - }}{\rm{M}}{{\rm{g}}^{\delta + }}{{\rm{X}}^{\delta - }}$.
The nucleophilic carbon centre makes it highly useful as we will see further in the reaction with acid halide.
An acid halide can be represented by a general formula \[{\rm{RCOX}}\] where a carboxylic proton has been replaced by a halogen. The presence of a carbonyl group helps in having an electrophilic carbon centre as $\left( {{\rm{C}} = {\rm{O}}} \right)$ bond is polar and electrons are pulled mostly by oxygen making carbon, electron deficient.
In a reaction of acid chloride with excess Grignard reagent, nucleophilic carbon will attack on the electrophilic and the subsequent hydrolysis will give an alcohol as follows:
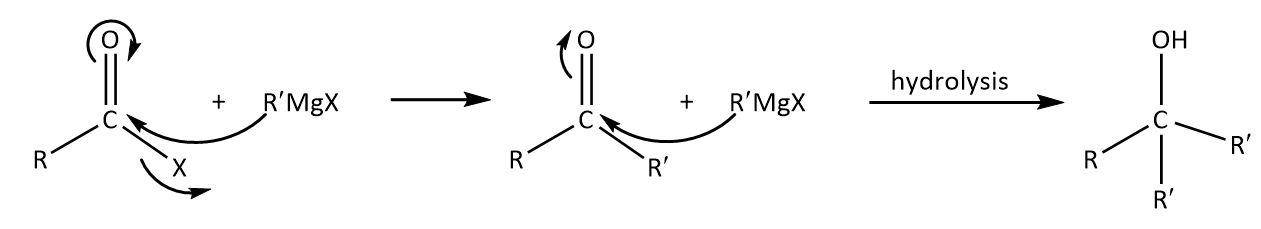
As we can see that the product contains two alkyl groups from the Grignard reagent that must be the same and one original that may or may not be the same.
Now, as in the given options, only one has the same substituents, in fact all of them are the same whereas all others have different substituents.
So, the product \[\left( {\rm{Y}} \right)\] can be:
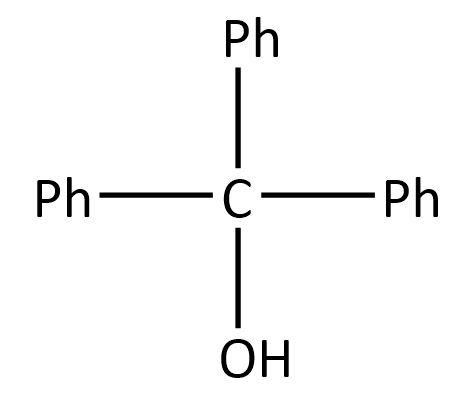
Hence, the correct option is A.
Note: The normal reaction of acid chloride with Grignard reagent would give ketone only; it is only when Grignard reagent is in excess that a tertiary alcohol is produced.
Step by step answer: Grignard reagent is an organo-metallic compound with a general formula ${\rm{RMgX}}$ in which an alkyl group $\left( {\rm{R}} \right)$ is attached to magnesium and there is a halide $\left( {\rm{X}} \right)$ present as well. In ${\rm{R}} - {\rm{MgX}}$ bond is covalent but due to Electronegativity difference, it is polar and ${\rm{RMg}} - {\rm{X}}$ bond is ionic in nature. This gives rise to charge separations as ${{\rm{R}}^{\delta - }}{\rm{M}}{{\rm{g}}^{\delta + }}{{\rm{X}}^{\delta - }}$.
The nucleophilic carbon centre makes it highly useful as we will see further in the reaction with acid halide.
An acid halide can be represented by a general formula \[{\rm{RCOX}}\] where a carboxylic proton has been replaced by a halogen. The presence of a carbonyl group helps in having an electrophilic carbon centre as $\left( {{\rm{C}} = {\rm{O}}} \right)$ bond is polar and electrons are pulled mostly by oxygen making carbon, electron deficient.
In a reaction of acid chloride with excess Grignard reagent, nucleophilic carbon will attack on the electrophilic and the subsequent hydrolysis will give an alcohol as follows:

As we can see that the product contains two alkyl groups from the Grignard reagent that must be the same and one original that may or may not be the same.
Now, as in the given options, only one has the same substituents, in fact all of them are the same whereas all others have different substituents.
So, the product \[\left( {\rm{Y}} \right)\] can be:

Hence, the correct option is A.
Note: The normal reaction of acid chloride with Grignard reagent would give ketone only; it is only when Grignard reagent is in excess that a tertiary alcohol is produced.
Recently Updated Pages
Master Class 10 Computer Science: Engaging Questions & Answers for Success

Master Class 10 General Knowledge: Engaging Questions & Answers for Success

Master Class 10 English: Engaging Questions & Answers for Success

Master Class 10 Social Science: Engaging Questions & Answers for Success

Master Class 10 Maths: Engaging Questions & Answers for Success

Master Class 10 Science: Engaging Questions & Answers for Success

Trending doubts
What is the median of the first 10 natural numbers class 10 maths CBSE

Which women's tennis player has 24 Grand Slam singles titles?

Who is the Brand Ambassador of Incredible India?

Why is there a time difference of about 5 hours between class 10 social science CBSE

Write a letter to the principal requesting him to grant class 10 english CBSE

A moving boat is observed from the top of a 150 m high class 10 maths CBSE




