
Among the triatomic molecules/ions,
$BeC{{l}_{2,}}{{N}_{3}}^{-},{{N}_{2}}O,N{{O}_{2}}^{+},{{O}_{3,}}SC{{l}_{2}},IC{{l}_{2}}^{-},{{I}_{3}}^{-}$and $Xe{{F}_{2}}$,the total number of linear molecule(s)/ion(s) (s) is
[Atomic number:$S=16,Cl=17,,I=53,Xe=54$]
Answer
590.4k+ views
Hint: Linear molecules have sp hybridization. Bond angle between atoms is 180.The hybridization of the central atom is determined by electronic configuration in ground state and electronic configuration in Excited state. When the total of electron pairs is 2, the hybridization of the central atom is sp.
Complete step by step answer:
-In $BeC{{l}_{2}}$, Be has electronic configuration as $1{{s}^{2}}2{{s}^{2}}$ As both electrons are paired in 2s , one electron from 2s is transferred to 2p. so, electronic configuration in an excited state is $1{{s}^{2}}2{{s}^{1}}2{{p}^{1}}$.
So, 2s and 2p orbitals undergo mixing to give sp hybridization, so bond angle is 180 degrees and geometry is linear.
-In ${{N}_{3}}^{-}$, Nitrogen has electronic configuration in excited state as $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$, it has linear structure.
-In ${{N}_{2}}O$, Nitrogen has two electron pairs and molecules have linear geometry.
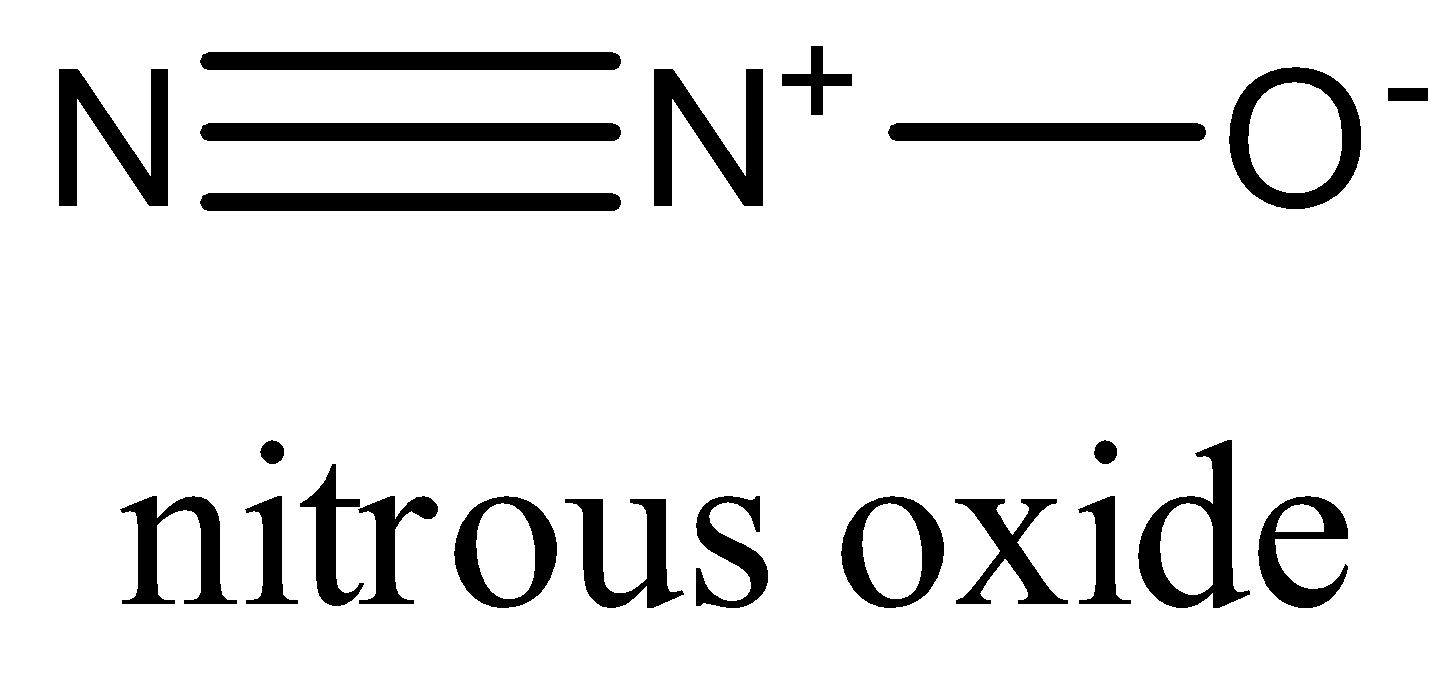
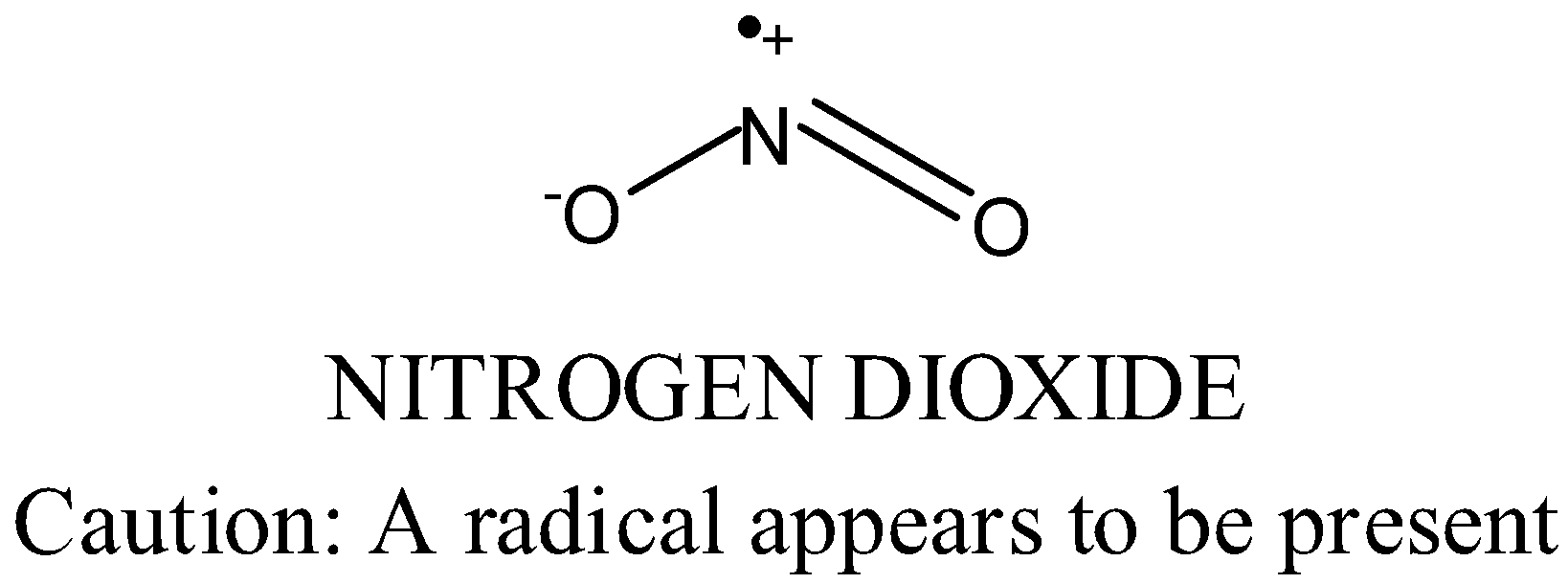
-In $N{{O}_{2}}^{+}$, Nitrogen loses on electrons and acquires positive charge and becomes linear.
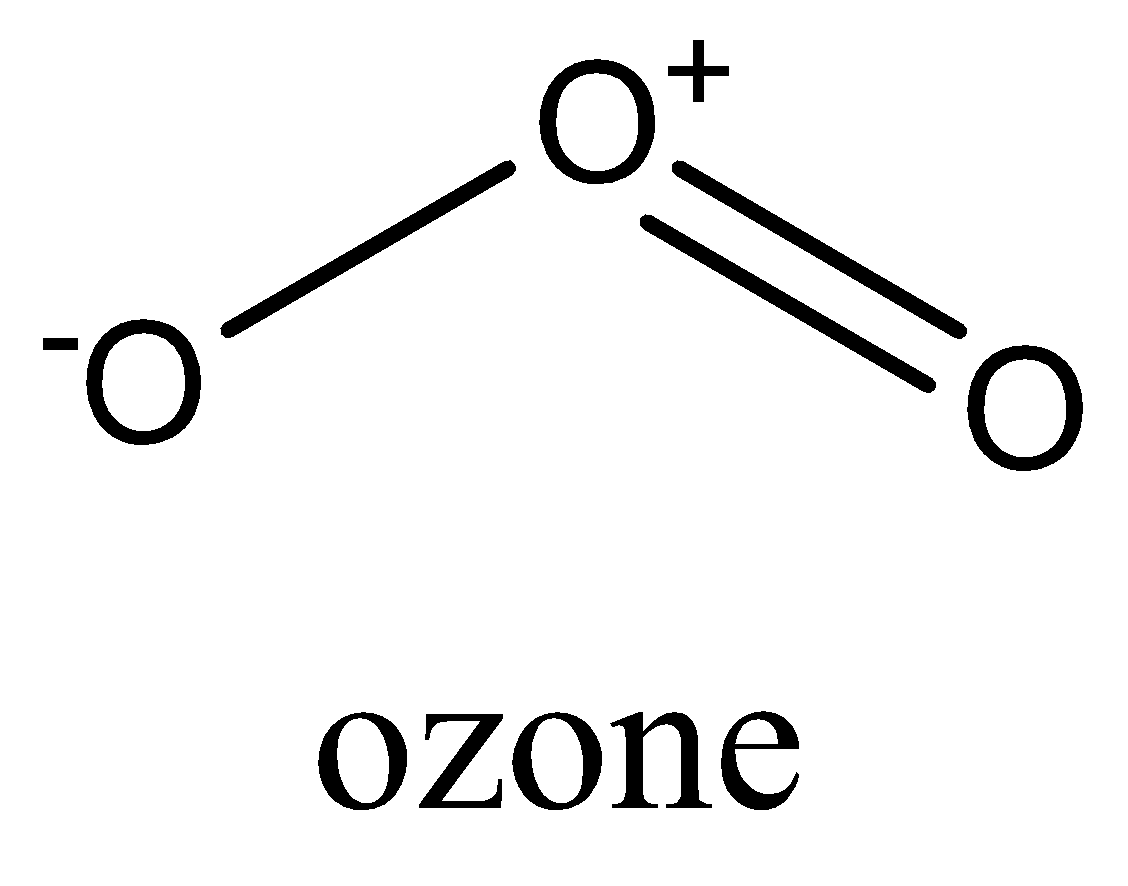
In ${{O}_{3}}$, the molecular has bent geometry.
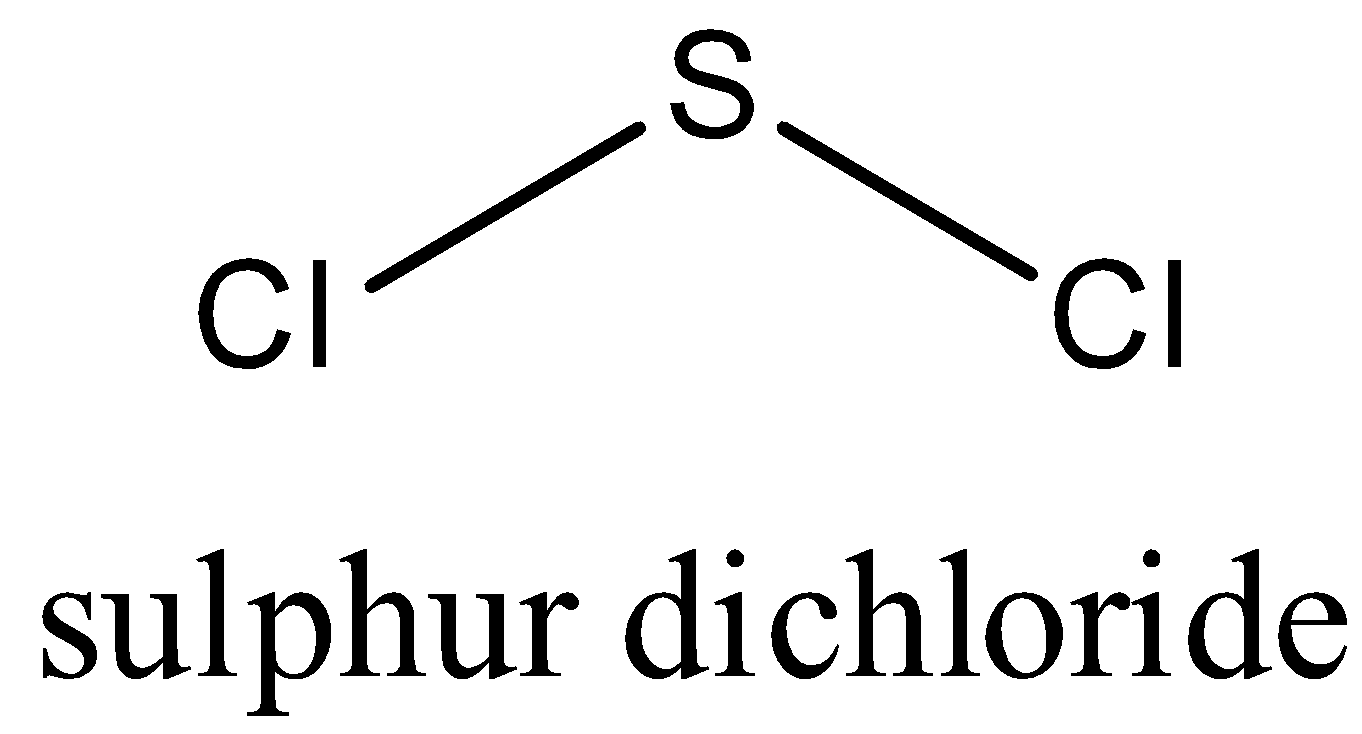
In $SC{{l}_{2}}$,Sulphur has two lone pairs of electrons so due to repulsion it has bent structure.
In $IC{{l}_{2}}^{-}$, Iodine has three lone pairs of electrons. Iodine has $s{{p}^{3}}d$ hybridization and trigonal bipyramidal geometry.

In ${{I}_{3}}^{-}$, iodine has three lone pairs of electrons, has $s{{p}^{3}}d$ hybridization, linear geometry with bond angle 180 degrees.
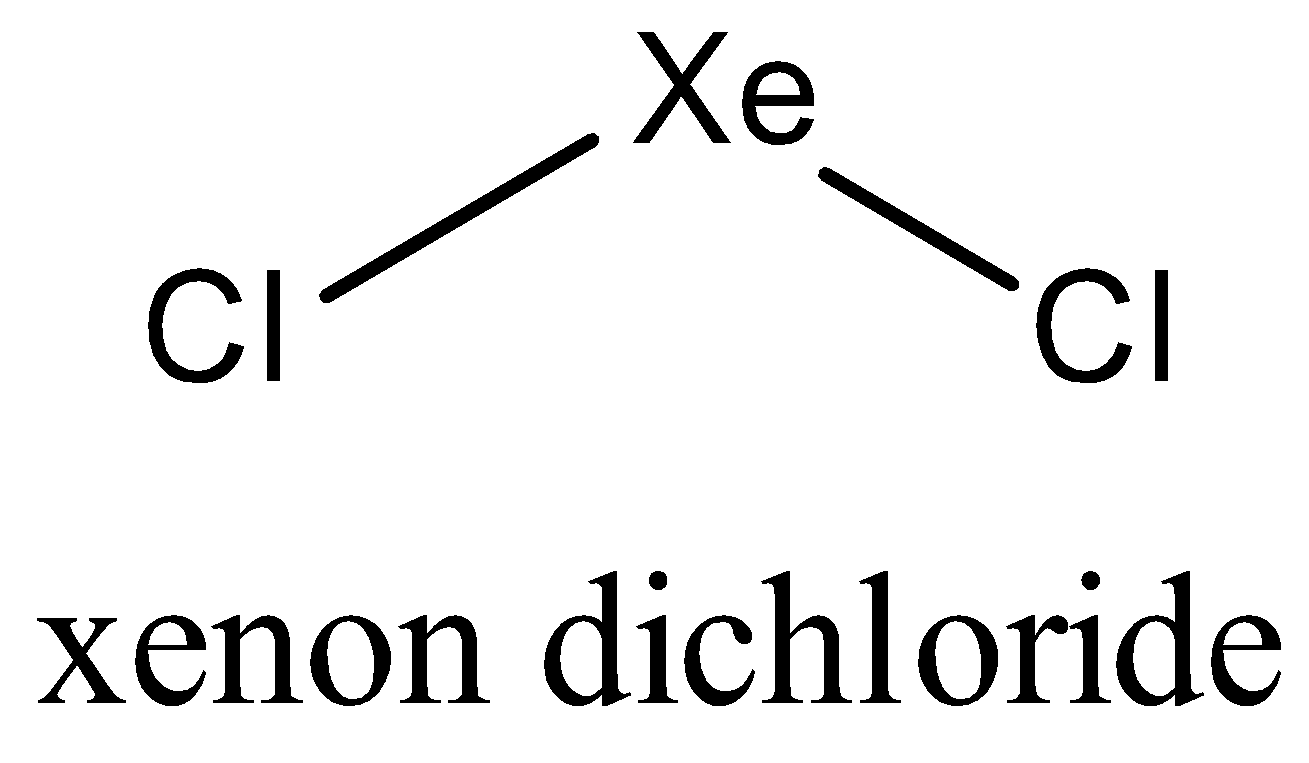
In $Xe{{F}_{2}}$,Xenon has three lone pairs of electrons .it has $s{{p}^{3}}d$ hybridization .Both Xe-F bonds make 180 degree bond with each other, so molecule is linear.
So, from the above explanation it can be concluded there are 4 molecules or ions having linear geometry where the hybridization of the central atom does not have contribution from the d-orbital.
Note: Hybridization can be determined by calculating total number of electron pairs which is sum of lone pairs and bonding pairs. when the total of electron pairs is five, hybridization is $s{{p}^{3}}d$.when total of electron pair is four ,hybridization is $s{{p}^{3}}$. When the total of electron pairs is three, hybridization is $s{{p}^{2}}$. When the total number of electron pairs is two, hybridization is sp. Halogens have Valence as one and three lone pairs of electrons are present . Nitrogen has one lone pair of electrons and oxygen has two lone pairs of electrons.
Complete step by step answer:
-In $BeC{{l}_{2}}$, Be has electronic configuration as $1{{s}^{2}}2{{s}^{2}}$ As both electrons are paired in 2s , one electron from 2s is transferred to 2p. so, electronic configuration in an excited state is $1{{s}^{2}}2{{s}^{1}}2{{p}^{1}}$.
So, 2s and 2p orbitals undergo mixing to give sp hybridization, so bond angle is 180 degrees and geometry is linear.
-In ${{N}_{3}}^{-}$, Nitrogen has electronic configuration in excited state as $1{{s}^{2}}2{{s}^{2}}2{{p}^{3}}$, it has linear structure.
-In ${{N}_{2}}O$, Nitrogen has two electron pairs and molecules have linear geometry.
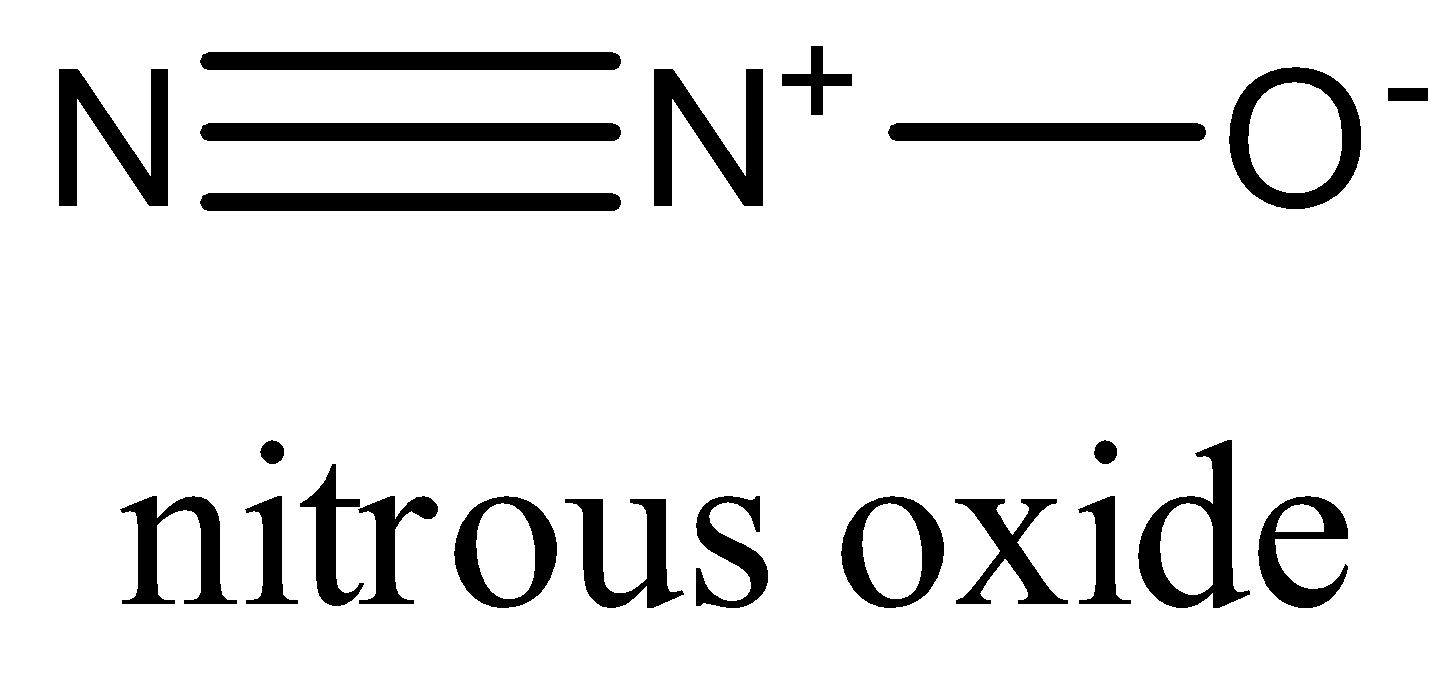
-In $N{{O}_{2}}^{+}$, Nitrogen loses on electrons and acquires positive charge and becomes linear.

In ${{O}_{3}}$, the molecular has bent geometry.

In $SC{{l}_{2}}$,Sulphur has two lone pairs of electrons so due to repulsion it has bent structure.

In $IC{{l}_{2}}^{-}$, Iodine has three lone pairs of electrons. Iodine has $s{{p}^{3}}d$ hybridization and trigonal bipyramidal geometry.

In ${{I}_{3}}^{-}$, iodine has three lone pairs of electrons, has $s{{p}^{3}}d$ hybridization, linear geometry with bond angle 180 degrees.
In $Xe{{F}_{2}}$,Xenon has three lone pairs of electrons .it has $s{{p}^{3}}d$ hybridization .Both Xe-F bonds make 180 degree bond with each other, so molecule is linear.

So, from the above explanation it can be concluded there are 4 molecules or ions having linear geometry where the hybridization of the central atom does not have contribution from the d-orbital.
Note: Hybridization can be determined by calculating total number of electron pairs which is sum of lone pairs and bonding pairs. when the total of electron pairs is five, hybridization is $s{{p}^{3}}d$.when total of electron pair is four ,hybridization is $s{{p}^{3}}$. When the total of electron pairs is three, hybridization is $s{{p}^{2}}$. When the total number of electron pairs is two, hybridization is sp. Halogens have Valence as one and three lone pairs of electrons are present . Nitrogen has one lone pair of electrons and oxygen has two lone pairs of electrons.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




