
Among the following, the lowest degree of paramagnetism per mole of the compound at 298 K will be shown by:
A.\[MnS{O_4}.4{H_2}O\]
B.\[CuS{O_4}.5{H_2}O\]
C.\[FeS{O_4}.6{H_2}O\]
D.\[NiS{O_4}.6{H_2}O\]
Answer
585.9k+ views
Hint: Crystal field theory helps to describe the breaking of energies of electron orbital states, due to another electric field produced by a surrounding charge distribution known as a ligand. This can describe many properties such as spectroscopies of transition metal coordination complexes, magnetic properties, colours, hydration enthalpies, and spinel structures of transition metal complexes.
Complete step by step answer:
Ligand means to tie or bond. It donates a pair of electrons to the central metal atom or ion to form a coordination complex.Ligands can be divided into strong field and weak field ligands. Strong field ligands produce large splitting in crystal field energy and weak field ligands produce a small splitting. As water is a weak field ligand, the configurations of metal ions in hydrated compounds resemble the electronic configuration in isolated gaseous ions as there is no pairing of electrons. All the metal ions in the above mentioned options are divalent and their outer shell electronic configurations can be written as
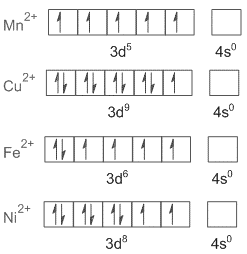
Now the question asks us about paramagnetism per mole. We know that the paramagnetic nature of a compound is directly proportional to the number of unpaired electrons in its electronic configuration. As we can see from the electronic configurations, $M{n^{2 + }}$ ion has a greater number of unpaired electrons. Hence \[MnS{O_4}.4{H_2}O\] shows greater paramagnetism. On the other hand, there is only one unpaired electron in $C{u^{2 + }}$and hence \[CuS{O_4}.5{H_2}O\;\] shows the lowest degree of paramagnetism.
Therefore, we can conclude that the correct answer to this question is option C. \[CuS{O_4}.5{H_2}O\;\]will show the lowest degree of paramagnetism per mole of the compound at 298 K.
Note:
Make sure that you remember the spectrochemical series of ligands. Here they are ordered by the size of the splitting crystal field energy:\[\begin{gathered}
{I^ - }\; < \;B{r^ - }\; < \;{S^{2 - }}\; < \;SC{N^ - }\;\left( {S-bonded} \right){\text{ }} < \;C{l^ - }\; < \;N{O_3}^ - \; < \;{N_3}^ - \; < \;{F^ - }\; < \;O{H^ - }\; < \;{C_2}{O_4}^{2 - }\; < \;{H_2}O\; < \;NC{S^ - }\;\left( {N-bonded} \right){\text{ }} < \; \\
C{H_3}CN\; < \;py\; < \;N{H_3}\; < \;en\; < \;2,2' - bipyridine\; < \;phen\; < \;N{O_2}^ - \; < \;PP{h_3}\; < \;C{N^ - }\; < \;CO. \\
\end{gathered} \]
Complete step by step answer:
Ligand means to tie or bond. It donates a pair of electrons to the central metal atom or ion to form a coordination complex.Ligands can be divided into strong field and weak field ligands. Strong field ligands produce large splitting in crystal field energy and weak field ligands produce a small splitting. As water is a weak field ligand, the configurations of metal ions in hydrated compounds resemble the electronic configuration in isolated gaseous ions as there is no pairing of electrons. All the metal ions in the above mentioned options are divalent and their outer shell electronic configurations can be written as

Now the question asks us about paramagnetism per mole. We know that the paramagnetic nature of a compound is directly proportional to the number of unpaired electrons in its electronic configuration. As we can see from the electronic configurations, $M{n^{2 + }}$ ion has a greater number of unpaired electrons. Hence \[MnS{O_4}.4{H_2}O\] shows greater paramagnetism. On the other hand, there is only one unpaired electron in $C{u^{2 + }}$and hence \[CuS{O_4}.5{H_2}O\;\] shows the lowest degree of paramagnetism.
Therefore, we can conclude that the correct answer to this question is option C. \[CuS{O_4}.5{H_2}O\;\]will show the lowest degree of paramagnetism per mole of the compound at 298 K.
Note:
Make sure that you remember the spectrochemical series of ligands. Here they are ordered by the size of the splitting crystal field energy:\[\begin{gathered}
{I^ - }\; < \;B{r^ - }\; < \;{S^{2 - }}\; < \;SC{N^ - }\;\left( {S-bonded} \right){\text{ }} < \;C{l^ - }\; < \;N{O_3}^ - \; < \;{N_3}^ - \; < \;{F^ - }\; < \;O{H^ - }\; < \;{C_2}{O_4}^{2 - }\; < \;{H_2}O\; < \;NC{S^ - }\;\left( {N-bonded} \right){\text{ }} < \; \\
C{H_3}CN\; < \;py\; < \;N{H_3}\; < \;en\; < \;2,2' - bipyridine\; < \;phen\; < \;N{O_2}^ - \; < \;PP{h_3}\; < \;C{N^ - }\; < \;CO. \\
\end{gathered} \]
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




