
Among the following free radical bromination reactions, select those in which $ {2^0} $ halide is the major product.
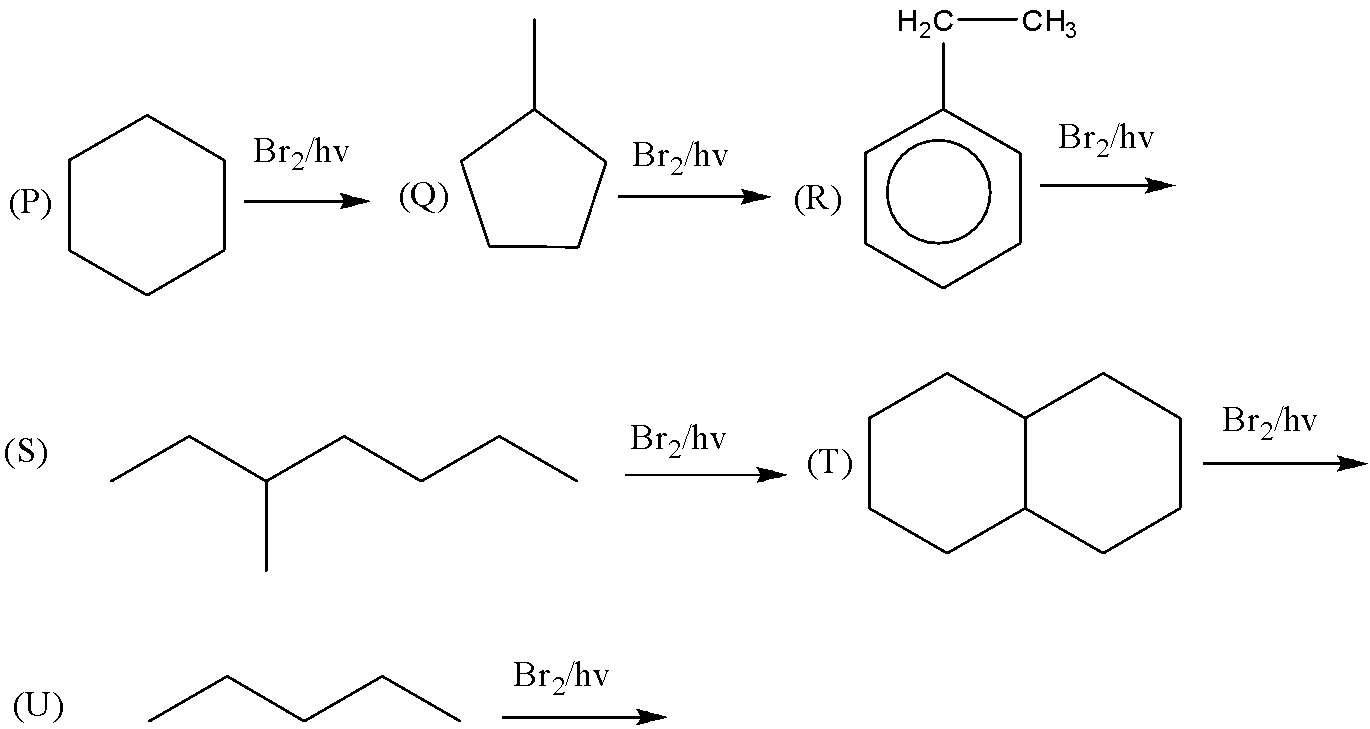
(A) $ P,Q,R,S $
(B) $ P,R,U $
(C) $ P,R,S,T $
(D) $ P,Q,R,S,T $

Answer
546.6k+ views
Hint: Here, we have given the free radical bromination reactions in which we will find the major product. Here we will find the reaction which forms $ {2^0} $ halide as the major product. Bromine is selective; it may form primary, secondary, or tertiary halide.
Complete step by step solution:
First, we will discuss the free radical bromination reactions. Free radical bromination reactions are the halogenation reactions that substitute chlorine or bromine for hydrogen on an alkane. Free radical halogenation reactions are performed in the presence of UV light. Now we will consider some reactions one by one which gives $ {2^0} $ halide as the major product. The $ {2^0} $ halide is the product formed when the carbon atom with the halogen group or any other group is attached with two more carbon atoms. So the reactions are as follows.
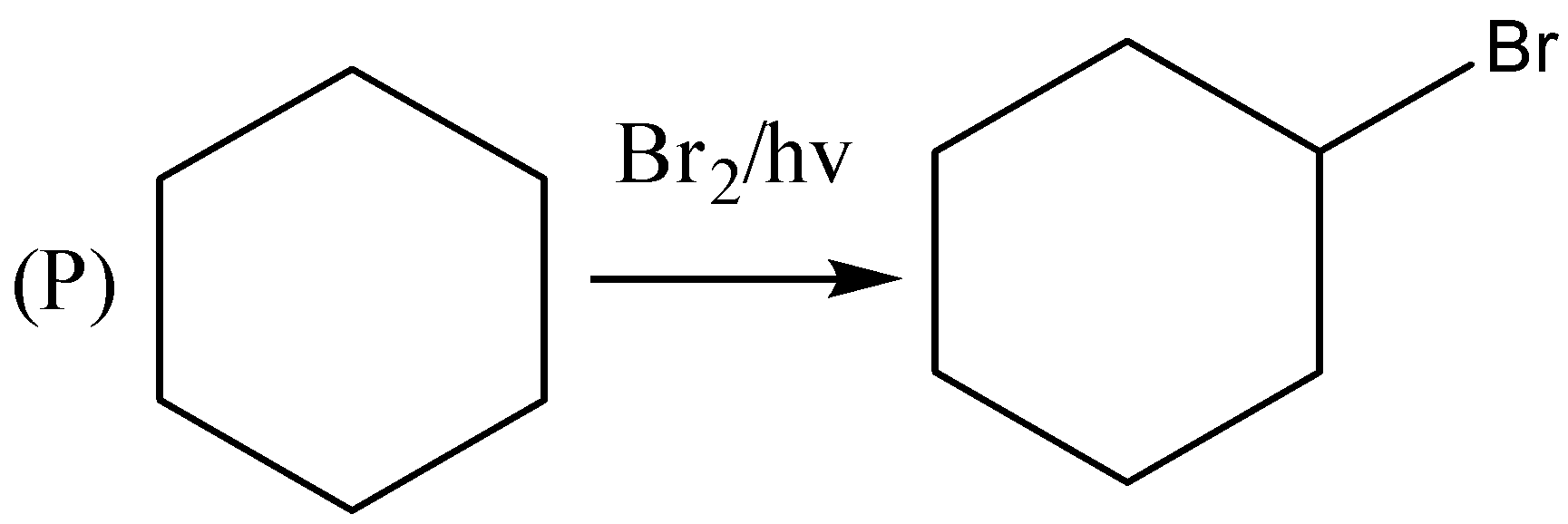
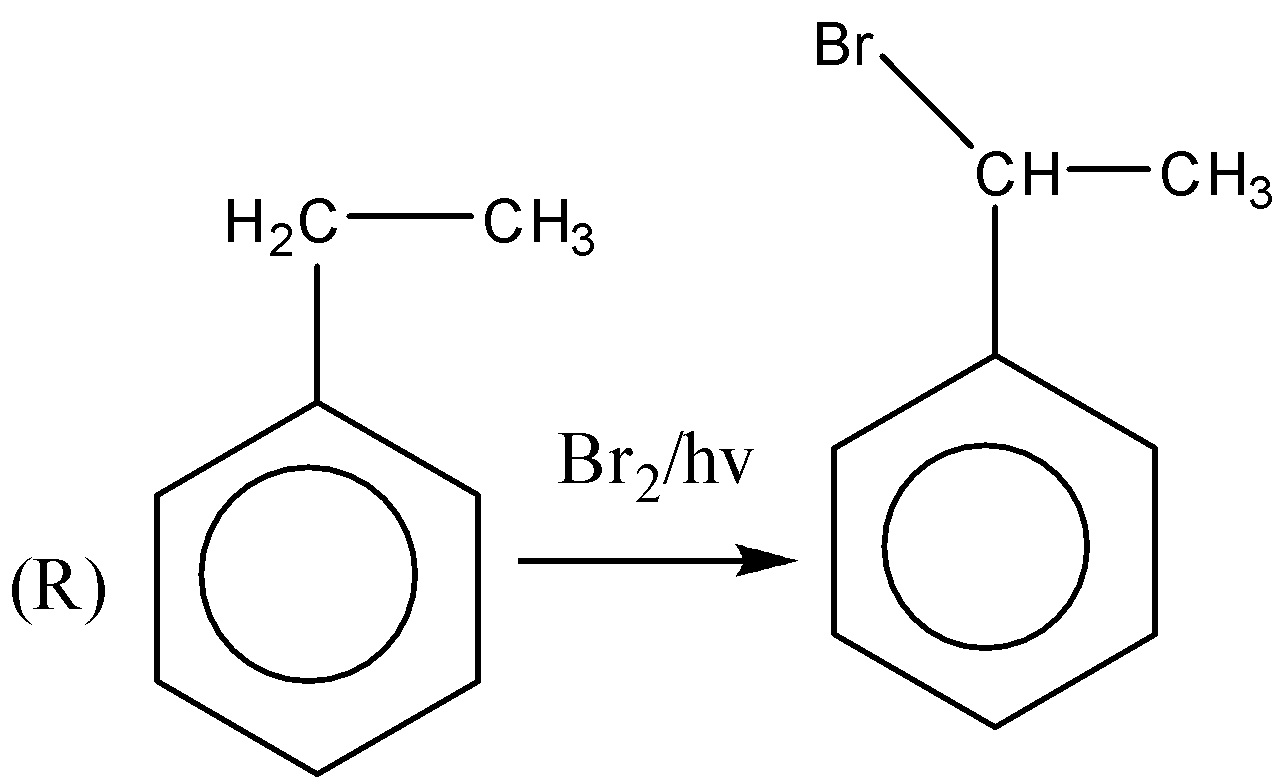
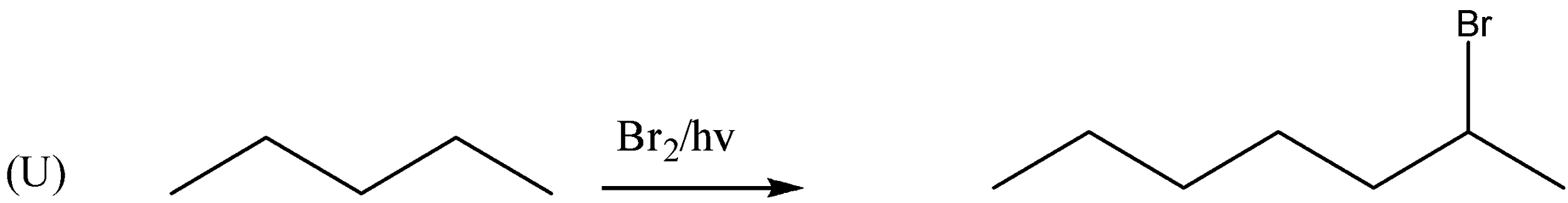
If we observe the major products formed by the reactions $ P,R,U $ . We can conclude that all the major products formed are $ {2^0} $ halide or secondary halide product. The carbon attached with bromine is surrounded by two carbon atoms. Therefore, the major product is $ {2^0} $ a halide. Similarly, $ (R) $ the carbon attached with bromine is attached with two carbon atoms. Therefore, the major product is $ {2^0} $ a halide. In reaction $ (U) $ also the major product formed is $ {2^0} $ a halide. In the reactions, $ Q,S,T $ the major products formed are $ {3^0} $ or tertiary halides.
Therefore, the correct option is (B).
Note:
Bromination is slower and more selective because a bromine atom is a less reactive hydrogen atom abstraction agent than a chlorine atom as reflected by the greater bond energy of $ HCl $ than $ HBr $ .
Complete step by step solution:
First, we will discuss the free radical bromination reactions. Free radical bromination reactions are the halogenation reactions that substitute chlorine or bromine for hydrogen on an alkane. Free radical halogenation reactions are performed in the presence of UV light. Now we will consider some reactions one by one which gives $ {2^0} $ halide as the major product. The $ {2^0} $ halide is the product formed when the carbon atom with the halogen group or any other group is attached with two more carbon atoms. So the reactions are as follows.



If we observe the major products formed by the reactions $ P,R,U $ . We can conclude that all the major products formed are $ {2^0} $ halide or secondary halide product. The carbon attached with bromine is surrounded by two carbon atoms. Therefore, the major product is $ {2^0} $ a halide. Similarly, $ (R) $ the carbon attached with bromine is attached with two carbon atoms. Therefore, the major product is $ {2^0} $ a halide. In reaction $ (U) $ also the major product formed is $ {2^0} $ a halide. In the reactions, $ Q,S,T $ the major products formed are $ {3^0} $ or tertiary halides.
Therefore, the correct option is (B).
Note:
Bromination is slower and more selective because a bromine atom is a less reactive hydrogen atom abstraction agent than a chlorine atom as reflected by the greater bond energy of $ HCl $ than $ HBr $ .
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




