
When acetone is condensed into a ketol the reagent used is:
A. ${\rm{Ba}}{\left( {{\rm{OH}}} \right)_2}$
B. ${\rm{NaHC}}{{\rm{O}}_{\rm{3}}}$
C. ${\rm{B}}{{\rm{r}}_{\rm{2}}}$ water
D. ${\rm{C}}{{\rm{l}}_{\rm{2}}}$
Answer
571.8k+ views
Hint: We know that, due to the acidic nature of $\alpha $ hydrogen of aldehyde and ketones, they undergo a number of reactions. The $\alpha $ hydrogen is the hydrogen bonded to the $\alpha $ carbon (carbon atom bonded to the functional group).
Complete step by step answer:
Let’s understand the aldol condensation reaction in detail. A ketone or aldehyde having at least one $\alpha $hydrogen undergo reaction in the presence of alkali (dilute) to form $\beta $-hydroxy ketone (keton)or $\beta $ -hydroxy aldehyde. This reaction is termed an aldol condensation reaction. One example is,
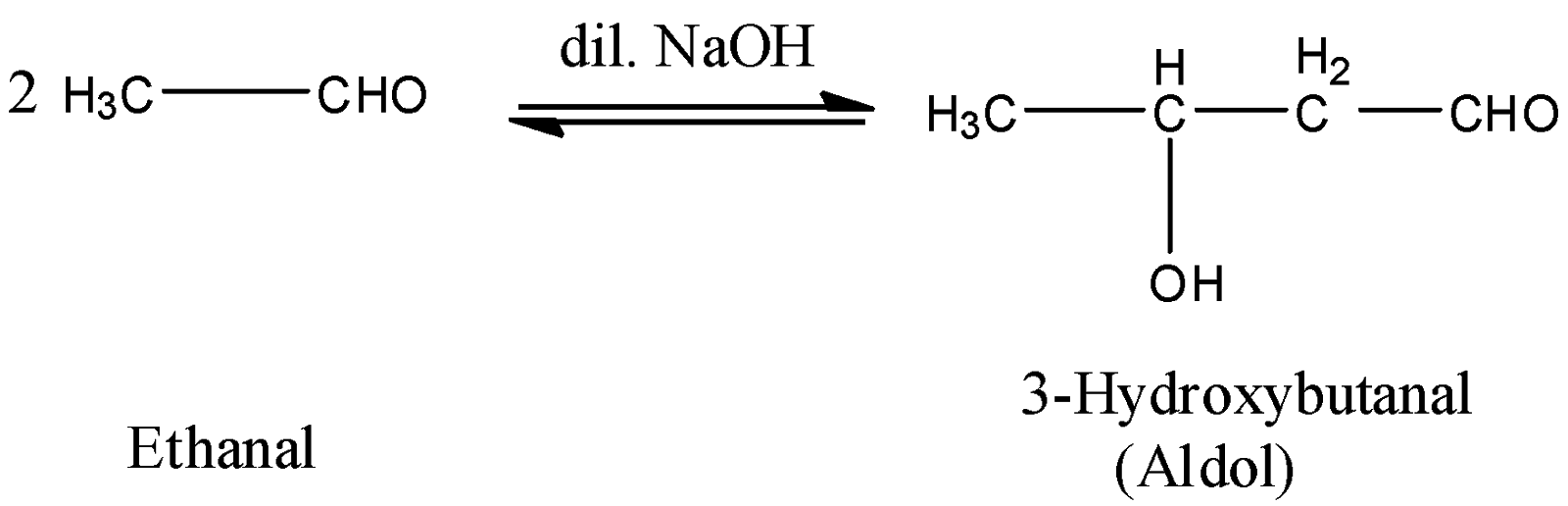
Now, come to the question. We have to identify the reagent used for the condensation of acetone. We know that, in the aldol condensation reaction, a base is used which acts as catalyst in the reaction. Barium hydroxide ${\rm{Ba}}{\left( {{\rm{OH}}} \right)_2}$ is the base. So, it should be used in the condensation reaction of acetone. The reaction of formation of ketol can be shown as below:
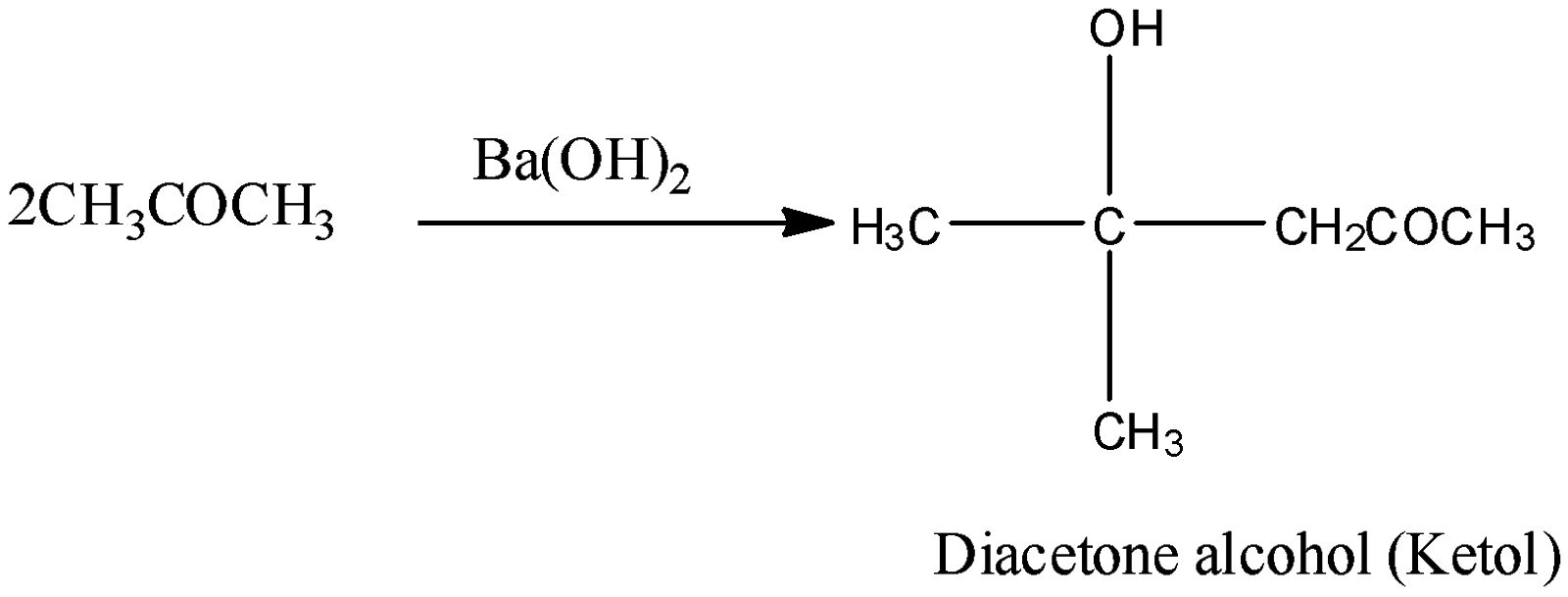
So, the correct answer is Option A.
Additional Information:
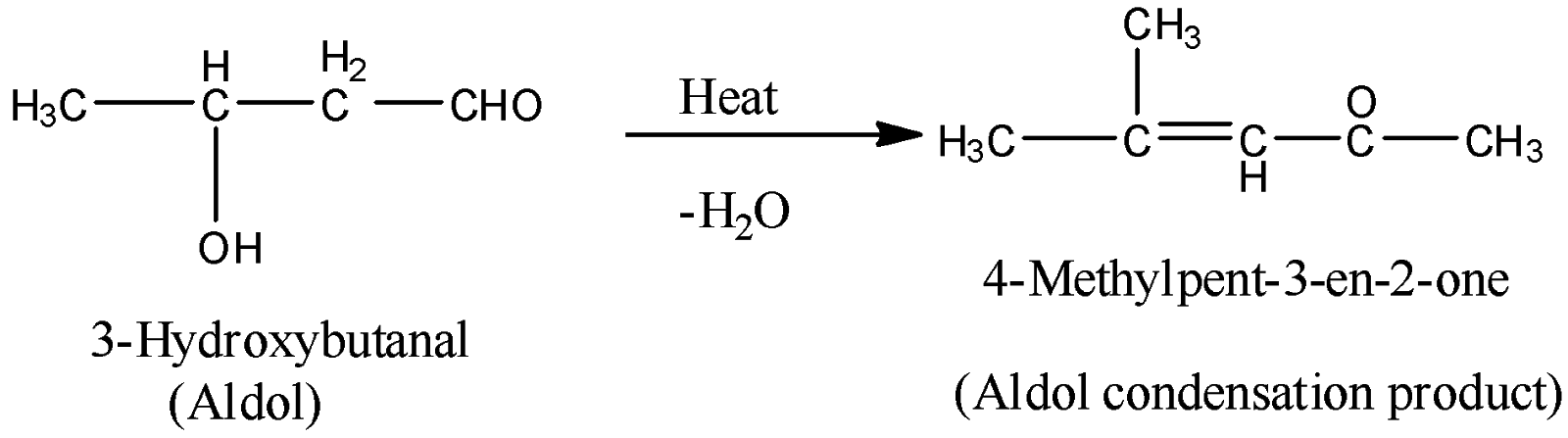
The products of the aldol condensation reactions are named as ketol or aldol because of the presence of two functional groups in the compound namely alcohol and aldehyde or ketone. The ketol and aldol easily lose water molecules to give $\alpha ,\beta $ unsaturated carbonyl compound (aldol condensation product). That’s why the reaction is termed as aldol condensation reaction.
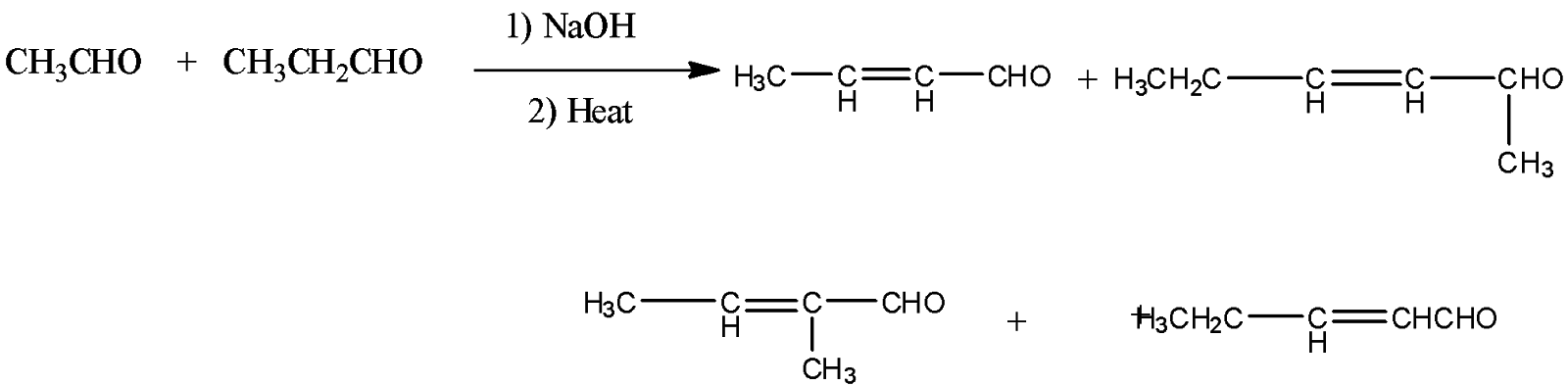
Note: Another type of aldol condensation reaction is cross aldol condensation. In this aldol condensation reaction, the two different aldehydes and ketones undergo reaction. If both the reactants consist of alpha hydrogen, then a mixture of four products is formed.
Complete step by step answer:
Let’s understand the aldol condensation reaction in detail. A ketone or aldehyde having at least one $\alpha $hydrogen undergo reaction in the presence of alkali (dilute) to form $\beta $-hydroxy ketone (keton)or $\beta $ -hydroxy aldehyde. This reaction is termed an aldol condensation reaction. One example is,
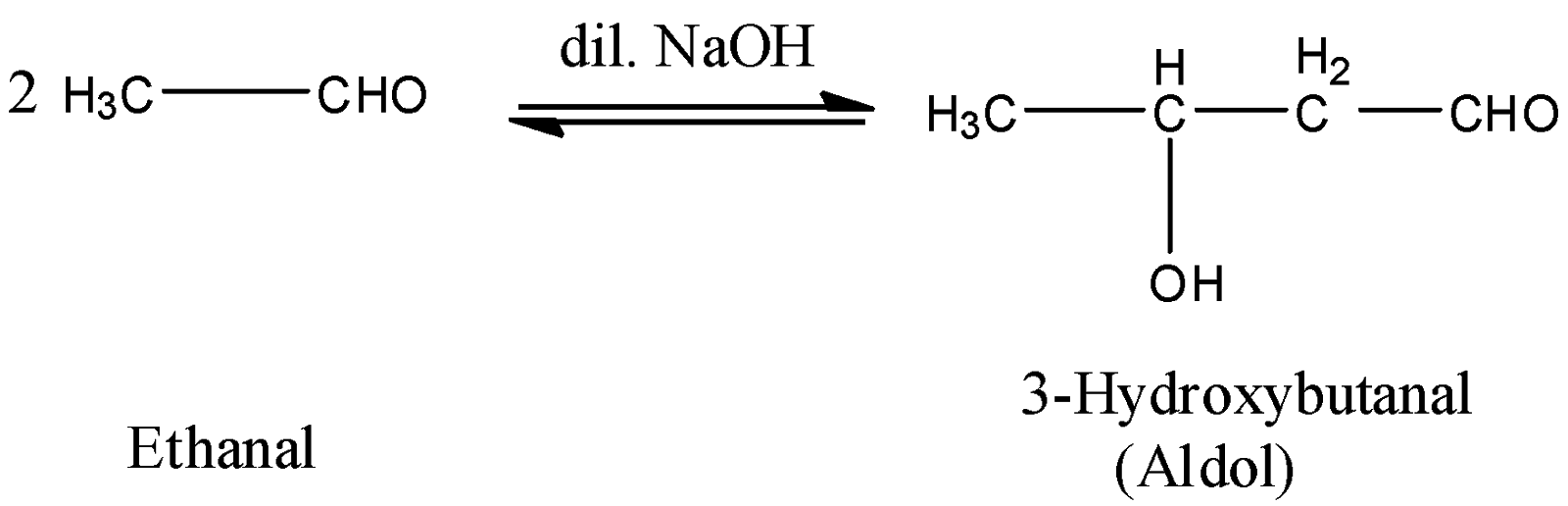
Now, come to the question. We have to identify the reagent used for the condensation of acetone. We know that, in the aldol condensation reaction, a base is used which acts as catalyst in the reaction. Barium hydroxide ${\rm{Ba}}{\left( {{\rm{OH}}} \right)_2}$ is the base. So, it should be used in the condensation reaction of acetone. The reaction of formation of ketol can be shown as below:

So, the correct answer is Option A.
Additional Information:
The products of the aldol condensation reactions are named as ketol or aldol because of the presence of two functional groups in the compound namely alcohol and aldehyde or ketone. The ketol and aldol easily lose water molecules to give $\alpha ,\beta $ unsaturated carbonyl compound (aldol condensation product). That’s why the reaction is termed as aldol condensation reaction.

Note: Another type of aldol condensation reaction is cross aldol condensation. In this aldol condensation reaction, the two different aldehydes and ketones undergo reaction. If both the reactants consist of alpha hydrogen, then a mixture of four products is formed.

Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




