
Acetate ion have:
(A) Two carbon, oxygen double bond
(B) Two carbon, oxygen single bond
(C) One carbon, oxygen single bond and one carbon, oxygen double bond
(D) None of these
Answer
576.3k+ views
Hint: Acetate ion is made up of two carbon atoms and two oxygen atoms. Its molecular formula is $C{H_3}CO{O^ - }$. A $\pi $-bond is formed between one carbon atom and one oxygen atom in acetate ions.
Complete step by step solution:
We will get some information about the acetate ions first in order to find which type of bonds are present in acetate ions.
- We know that we use –oic acid suffix to show the carboxylic acid group (-COOH). Now, what would we call a compound if it contains $ - CO{O^ - }$ group?
- The compound that contains $ - CO{O^ - }$ groups is generally named by using the suffix –ate.
- Acetate shows that the number of carbons present in the compound is two.
- We know that carbon atoms are tetravalent atoms. Oxygen atoms can form two bonds normally. So, we can say that the structure of acetate ions is as shown below.
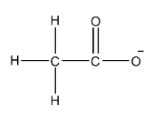
- Here, we can see that one methyl group is attached to the carbonyl carbon. The carbonyl carbon is the carbon which forms a double bond with oxygen atom. The carbonyl carbon also forms a single bond with an oxygen atom.
Thus, we can say that acetate ions has One carbon, oxygen single bond and one carbon, oxygen double bond
So, the correct answer is (C).
Note: Note that acetate ion is the conjugate base of acetic acid. Thus, they are basic in nature. They are produced in the solution of acetic acid if any base is added to the solution. The negative charge is shifted from one oxygen atom to another oxygen atom by resonance.
Complete step by step solution:
We will get some information about the acetate ions first in order to find which type of bonds are present in acetate ions.
- We know that we use –oic acid suffix to show the carboxylic acid group (-COOH). Now, what would we call a compound if it contains $ - CO{O^ - }$ group?
- The compound that contains $ - CO{O^ - }$ groups is generally named by using the suffix –ate.
- Acetate shows that the number of carbons present in the compound is two.
- We know that carbon atoms are tetravalent atoms. Oxygen atoms can form two bonds normally. So, we can say that the structure of acetate ions is as shown below.

- Here, we can see that one methyl group is attached to the carbonyl carbon. The carbonyl carbon is the carbon which forms a double bond with oxygen atom. The carbonyl carbon also forms a single bond with an oxygen atom.
Thus, we can say that acetate ions has One carbon, oxygen single bond and one carbon, oxygen double bond
So, the correct answer is (C).
Note: Note that acetate ion is the conjugate base of acetic acid. Thus, they are basic in nature. They are produced in the solution of acetic acid if any base is added to the solution. The negative charge is shifted from one oxygen atom to another oxygen atom by resonance.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




