
Acetaldehyde reacts with semicarbazide, product will be:
A.\[C{{H}_{3}}CH=NNH-CO-N{{H}_{2}}\]
B.\[C{{H}_{3}}CH=NCONHN{{H}_{2}}\]
C.\[C{{H}_{3}}CH=NHN{{H}_{2}}\]
D.\[C{{H}_{3}}-C\left( =O \right)-NH-CON{{H}_{2}}\]
Answer
526.2k+ views
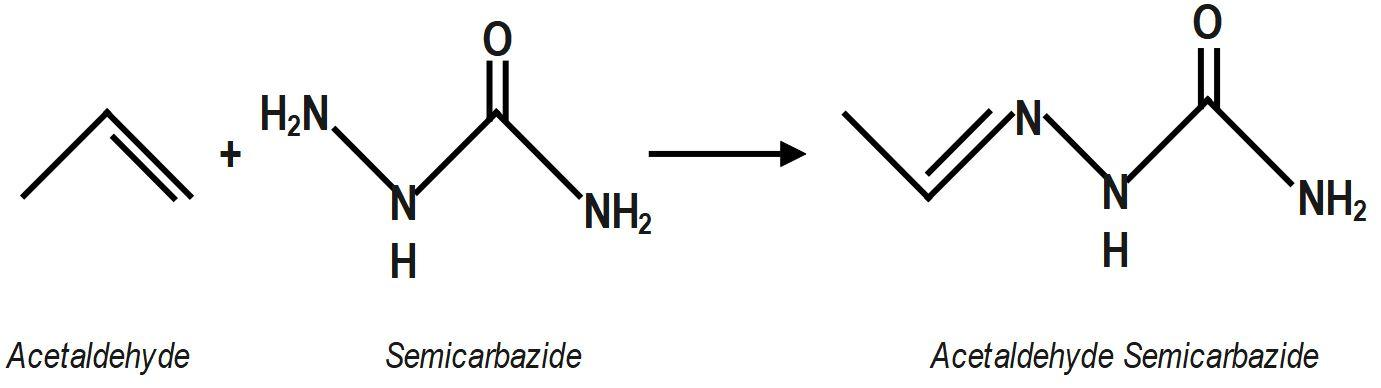
Hint: We know that Semicarbazone are derivatives of imines and formed by the condensation reaction between an aldehyde or ketone and semicarbazide. Chemical formula of semicarbazide is $N{{H}_{2}}(CONH)N{{H}_{2}}$ the condensation reaction between acetaldehyde $\left( C{{H}_{3}}CHO \right)$ and semicarbazide to get the molecular formula of acetaldehyde semicarbazone.
Complete answer:
Acetaldehyde semicarbazone belongs to the group of semicarbazones. Semicarbazones are formed by the condensation reaction between an aldehyde or ketone and semicarbazide. A condensation reaction proceeds with the loss of a water molecule. So, to form acetaldehyde semicarbazone, we need to do the condensation reaction of aldehyde, which is acetaldehyde here, with semicarbazide. Semicarbazide is a derivative of urea.
Some semicarbazones have antiseptic properties. Nitrofurazone (trade name as Furacin) is such an example. Thiosemicarbazone is a semicarbazone which contains sulphur atoms (thio group) in the place of oxygen atoms. It possesses anti-viral, anti-malarial and anti-cancer activities.
Therefore, the correct answer is option A.
Note:
Remember that Semicarbazones are crystalline solids and they are very useful for the identification of aldehydes and ketones by the melting point analysis method. Molecular weight of acetaldehyde semicarbazone is \[101\text{ }g/mol.\] Its molecular formula can also be written as: ${{C}_{3}}{{H}_{7}}{{N}_{3}}O.$
Complete answer:
Acetaldehyde semicarbazone belongs to the group of semicarbazones. Semicarbazones are formed by the condensation reaction between an aldehyde or ketone and semicarbazide. A condensation reaction proceeds with the loss of a water molecule. So, to form acetaldehyde semicarbazone, we need to do the condensation reaction of aldehyde, which is acetaldehyde here, with semicarbazide. Semicarbazide is a derivative of urea.

Some semicarbazones have antiseptic properties. Nitrofurazone (trade name as Furacin) is such an example. Thiosemicarbazone is a semicarbazone which contains sulphur atoms (thio group) in the place of oxygen atoms. It possesses anti-viral, anti-malarial and anti-cancer activities.
Therefore, the correct answer is option A.
Note:
Remember that Semicarbazones are crystalline solids and they are very useful for the identification of aldehydes and ketones by the melting point analysis method. Molecular weight of acetaldehyde semicarbazone is \[101\text{ }g/mol.\] Its molecular formula can also be written as: ${{C}_{3}}{{H}_{7}}{{N}_{3}}O.$
Recently Updated Pages
Basicity of sulphurous acid and sulphuric acid are

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




