
Accomplish the conversion of aniline to \[2,4,6 - \]tribromofluorobenzene.
Answer
517.8k+ views
Hint: We have to know that the heat in a chemical reaction is either added or released during chemical reaction. Exothermic reactions are the one in which heat is released in a reaction whereas if heat is absorbed in a chemical reaction then it is an endothermic reaction.
Complete answer:
We have to remember that the conversion of aniline to \[2,4,6 - \]tribromofluorobenzene can be seen below. The conversion can be represented as:
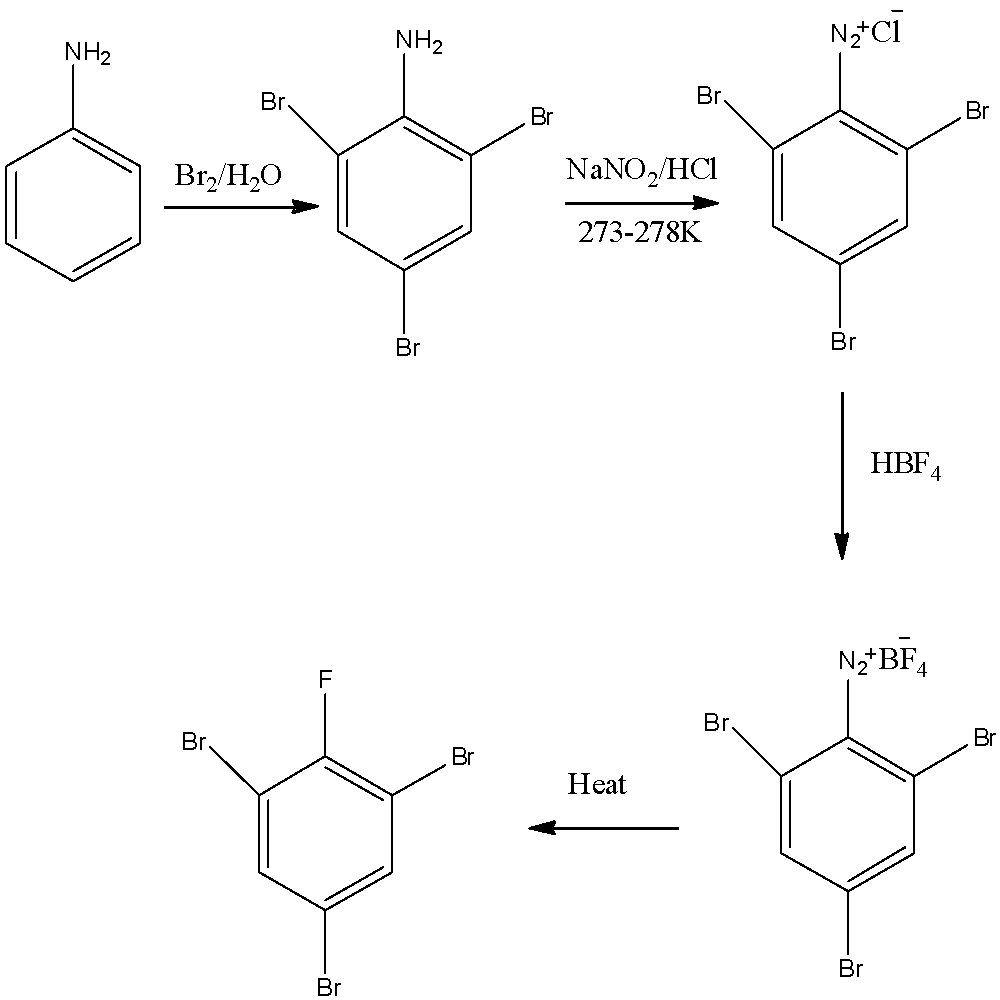
Aniline is an organic compound containing a benzene ring and an aniline group attached to that ring. Aniline is first treated with bromine and water; the product so formed is \[2,4,6 - \]tribromoaniline as the bromine groups attack on ortho and para positions. On further to get dinitrochloride salt we treat \[2,4,6 - \]tribromoaniline with sodium nitrate and hydrochloric acid to get amine group dissociate into ions on reacting with an acid, the product so formed is \[2,4,6 - \]tribromobenzene diazonium chloride further we will react this salt so formed with tetrafluoroboric acid , it is adding in order to replace chloride ion with tetrafluoroborate and finally on heating the ions gets removed and we only left with fluorine atom occupying the position on which salt was formed. From starting to end the conversion of aniline to \[2,4,6 - \]tribromofluorobenzene is formed.
Note:
When we add bromine to any reaction, the reaction can be termed as bromination reaction. When aniline is reduced to its salt on reacting with sodium nitrate and hydrochloric acid a diazonium salt is formed, this type of reaction or mechanism is known as diazotization reaction. Tetrafluoroboric acid is a Lewis acid as it can accept a pair of electrons easily.
Complete answer:
We have to remember that the conversion of aniline to \[2,4,6 - \]tribromofluorobenzene can be seen below. The conversion can be represented as:

Aniline is an organic compound containing a benzene ring and an aniline group attached to that ring. Aniline is first treated with bromine and water; the product so formed is \[2,4,6 - \]tribromoaniline as the bromine groups attack on ortho and para positions. On further to get dinitrochloride salt we treat \[2,4,6 - \]tribromoaniline with sodium nitrate and hydrochloric acid to get amine group dissociate into ions on reacting with an acid, the product so formed is \[2,4,6 - \]tribromobenzene diazonium chloride further we will react this salt so formed with tetrafluoroboric acid , it is adding in order to replace chloride ion with tetrafluoroborate and finally on heating the ions gets removed and we only left with fluorine atom occupying the position on which salt was formed. From starting to end the conversion of aniline to \[2,4,6 - \]tribromofluorobenzene is formed.
Note:
When we add bromine to any reaction, the reaction can be termed as bromination reaction. When aniline is reduced to its salt on reacting with sodium nitrate and hydrochloric acid a diazonium salt is formed, this type of reaction or mechanism is known as diazotization reaction. Tetrafluoroboric acid is a Lewis acid as it can accept a pair of electrons easily.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




