
(a)Account for the following:
(i) $Cl-C{{H}_{2}}COOH$ is a stronger acid than $C{{H}_{3}}COOH$
(ii) Carboxylic acids do not give reactions of carbonyl groups.
(b) Write the chemical equation to illustrate the following name reactions:
(i) Rosenmund reduction
(ii) Cannizzaro’s reaction
(c) Out of $C{{H}_{3}}C{{H}_{2}}-CO-C{{H}_{3}}$ and $C{{H}_{3}}C{{H}_{2}}-C{{H}_{2}}-CO-C{{H}_{3}}$, which give iodoform test?
Answer
548.1k+ views
Hint:The inductive effect is an effect in which a substituent which is attached to the original group, attracts the electron density towards itself. Such cases are said to have a negative inductive effect.
Whereas, positive inductive effect has substituents which pushes away the electron density from themselves.
Complete step-by-step answer: This question should be answered in a systematic manner by taking one part of the question at a time.
(a) The first question has two subparts.
(i) This question could be answered by using the concept of inductive effect. The chlorine atom shows negative inductive effect which can be identified by the pulling of the electron clouds towards the substituent group which is the chlorine in this case. So, the compound $Cl-C{{H}_{2}}COOH$ will show a negative inductive effect. While in case of acetic acid, due to availability of methyl group it shows positive inductive effect. The electron density which is observed in the case of $O-H$ bond in the compound $Cl-C{{H}_{2}}COOH$ is lesser because of the negative inductive effect and so the bond is much weaker while in case of acetic acid the hydroxide bond is stronger and so the loss of electron in acetic acid is harder than the loss of proton in $Cl-C{{H}_{2}}COOH$. Hence, $Cl-C{{H}_{2}}COOH$ is a stronger acid than $C{{H}_{3}}COOH$.
(ii) The resonating structures of carboxylic acid are as follows,
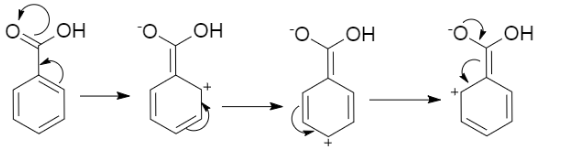
Just like the resonating structures of carboxylic acid, there exists resonating structures of other carbonyl groups which are ketones and aldehydes. The carbonyl carbon or the carbon which has a double bond with the oxygen in an aldehyde or ketone are electrophilic in nature. Now, if we consider the electrophilic character of the considered molecule of the carboxylic acid, it is reduced due to the presence of resonating structures. As the carbonyl carbon of the carboxylic group is more electronegative than the carbonyl compound of the aldehyde and ketone group therefore the carboxylic acid does not show nucleophilic addition reaction of the ketones and aldehyde.
(b) In the next question, there are again two subparts.
(i) The first one asks about rosenmund reduction. It is a type of reaction where acid chlorides get converted to their respective aldehydes by the use of hydrogen in the palladium and the barium sulphate is used as the poison catalyst. The general reaction can be represented as shown below,
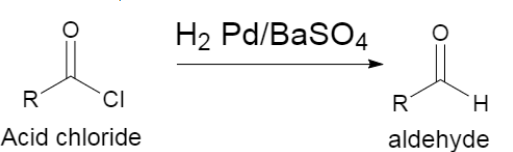
Here as we can see the chloride group is reduced in order to form a hydrogen group giving rise to the formation of an aldehyde group, as a result.
(ii) In the next part we are supposed to write the cannizaro’s reaction, which involves the disproportionation reaction of two molecules of aldehyde which does not contain any alpha hydrogens. This reaction is base induced, and as an end product it gives rise to a molecule of carboxylic acid and a primary alcohol. The reaction can be represented as shown below,
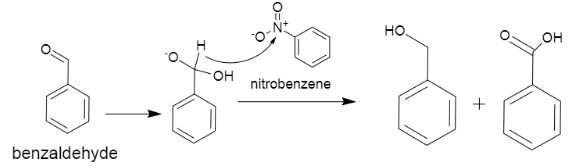
As we can see in the case of benzaldehyde, it produces a benzoic acid and an alcoholic compound.
(c) Iodoform tests are specifically given by the compounds which contain $-OC{{H}_{3}}$ groups. Among the given two species, only $C{{H}_{3}}C{{H}_{2}}-CO-C{{H}_{3}}$ contains a methoxy group and so it will give iodoform test.
Note:Cannizzaro’s reactions are only applicable for the aldehydes which do not contain any alpha hydrogens. Rosenmund reduction is a type of reduction which converts acyl chlorides to aldehydes.
The iodoform test is given by the compounds which contain a methoxy group. The absence of a methoxy group would result in an unreactive response.
Whereas, positive inductive effect has substituents which pushes away the electron density from themselves.
Complete step-by-step answer: This question should be answered in a systematic manner by taking one part of the question at a time.
(a) The first question has two subparts.
(i) This question could be answered by using the concept of inductive effect. The chlorine atom shows negative inductive effect which can be identified by the pulling of the electron clouds towards the substituent group which is the chlorine in this case. So, the compound $Cl-C{{H}_{2}}COOH$ will show a negative inductive effect. While in case of acetic acid, due to availability of methyl group it shows positive inductive effect. The electron density which is observed in the case of $O-H$ bond in the compound $Cl-C{{H}_{2}}COOH$ is lesser because of the negative inductive effect and so the bond is much weaker while in case of acetic acid the hydroxide bond is stronger and so the loss of electron in acetic acid is harder than the loss of proton in $Cl-C{{H}_{2}}COOH$. Hence, $Cl-C{{H}_{2}}COOH$ is a stronger acid than $C{{H}_{3}}COOH$.
(ii) The resonating structures of carboxylic acid are as follows,

Just like the resonating structures of carboxylic acid, there exists resonating structures of other carbonyl groups which are ketones and aldehydes. The carbonyl carbon or the carbon which has a double bond with the oxygen in an aldehyde or ketone are electrophilic in nature. Now, if we consider the electrophilic character of the considered molecule of the carboxylic acid, it is reduced due to the presence of resonating structures. As the carbonyl carbon of the carboxylic group is more electronegative than the carbonyl compound of the aldehyde and ketone group therefore the carboxylic acid does not show nucleophilic addition reaction of the ketones and aldehyde.
(b) In the next question, there are again two subparts.
(i) The first one asks about rosenmund reduction. It is a type of reaction where acid chlorides get converted to their respective aldehydes by the use of hydrogen in the palladium and the barium sulphate is used as the poison catalyst. The general reaction can be represented as shown below,
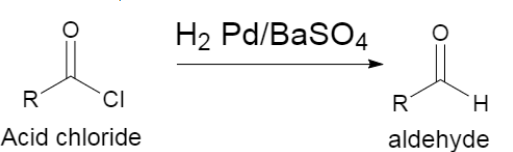
Here as we can see the chloride group is reduced in order to form a hydrogen group giving rise to the formation of an aldehyde group, as a result.
(ii) In the next part we are supposed to write the cannizaro’s reaction, which involves the disproportionation reaction of two molecules of aldehyde which does not contain any alpha hydrogens. This reaction is base induced, and as an end product it gives rise to a molecule of carboxylic acid and a primary alcohol. The reaction can be represented as shown below,

As we can see in the case of benzaldehyde, it produces a benzoic acid and an alcoholic compound.
(c) Iodoform tests are specifically given by the compounds which contain $-OC{{H}_{3}}$ groups. Among the given two species, only $C{{H}_{3}}C{{H}_{2}}-CO-C{{H}_{3}}$ contains a methoxy group and so it will give iodoform test.
Note:Cannizzaro’s reactions are only applicable for the aldehydes which do not contain any alpha hydrogens. Rosenmund reduction is a type of reduction which converts acyl chlorides to aldehydes.
The iodoform test is given by the compounds which contain a methoxy group. The absence of a methoxy group would result in an unreactive response.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




