
(a) Write the mechanism of the following reaction.
${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\xrightarrow{{{\text{HBr}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} + {{\text{H}}_{\text{2}}}{\text{O}}$
(b) Write the equation involved in the Reimer-Tiemann reaction.
Answer
558.9k+ views
Hint:In the first part we are given a reaction in which ethyl alcohol reacts with hydrogen bromide. In the reaction, an electron rich compound replaces the leaving group. To solve the second part we must know that in the Reimer-Tiemann reaction phenol is converted to an ortho-hydroxybenzaldehyde.
Complete step-by-step answer:(a) We are given the reaction as follows:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\xrightarrow{{{\text{HBr}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} + {{\text{H}}_{\text{2}}}{\text{O}}$
In the given reaction, ethanol i.e. alcohol reacts with hydrogen bromide. The product formed is ethyl hydrogen bromide which is an alkyl halide along with water which is the by-product of the reaction.
In the reaction, an electron rich compound i.e. the bromine atom replaces the hydroxyl leaving group.
The reactions in which an electron rich compound replaces the leaving group is known as nucleophilic substitution reaction.
The mechanism of the reaction is as follows:
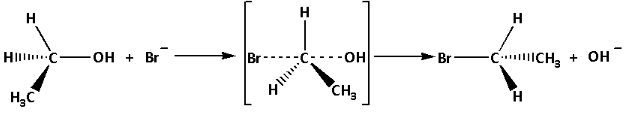
In the reaction mechanism, we can see that the simultaneous breaking and making of the bond occurs. The configuration is inverted. Thus, the mechanism of the reaction is ${{\text{S}}_{\text{N}}}{\text{2}}$ i.e. nucleophilic substitution bimolecular mechanism.
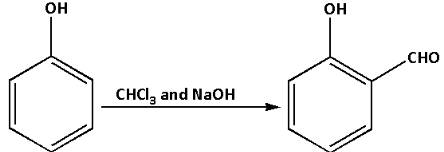
(b) In the Reimer-Tiemann reaction, phenol is converted to an ortho hydroxybenzaldehyde. It is a type of substitution reaction.In the Reimer-Tiemann reaction, phenol is treated with chloroform in the presence of sodium hydroxide. Thus, an aldehyde group is introduced at the ortho-position of the benzene ring. This leads to the formation of ortho-hydroxybenzaldehyde.
The equation for the Reimer-Tiemann reaction is as follows:
Note:The ${{\text{S}}_{\text{N}}}{\text{2}}$ reaction is a nucleophilic substitution reaction in which bond breaking and bond formation occurs simultaneously. The ${{\text{S}}_{\text{N}}}{\text{2}}$ reaction mechanism requires the attack of nucleophile from the back side of the carbon atom. Thus, the product obtained has the configuration opposite to that of the reactant. This is known as inversion of configuration.
Complete step-by-step answer:(a) We are given the reaction as follows:
${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}\xrightarrow{{{\text{HBr}}}}{\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{Br}} + {{\text{H}}_{\text{2}}}{\text{O}}$
In the given reaction, ethanol i.e. alcohol reacts with hydrogen bromide. The product formed is ethyl hydrogen bromide which is an alkyl halide along with water which is the by-product of the reaction.
In the reaction, an electron rich compound i.e. the bromine atom replaces the hydroxyl leaving group.
The reactions in which an electron rich compound replaces the leaving group is known as nucleophilic substitution reaction.
The mechanism of the reaction is as follows:

In the reaction mechanism, we can see that the simultaneous breaking and making of the bond occurs. The configuration is inverted. Thus, the mechanism of the reaction is ${{\text{S}}_{\text{N}}}{\text{2}}$ i.e. nucleophilic substitution bimolecular mechanism.
(b) In the Reimer-Tiemann reaction, phenol is converted to an ortho hydroxybenzaldehyde. It is a type of substitution reaction.In the Reimer-Tiemann reaction, phenol is treated with chloroform in the presence of sodium hydroxide. Thus, an aldehyde group is introduced at the ortho-position of the benzene ring. This leads to the formation of ortho-hydroxybenzaldehyde.
The equation for the Reimer-Tiemann reaction is as follows:

Note:The ${{\text{S}}_{\text{N}}}{\text{2}}$ reaction is a nucleophilic substitution reaction in which bond breaking and bond formation occurs simultaneously. The ${{\text{S}}_{\text{N}}}{\text{2}}$ reaction mechanism requires the attack of nucleophile from the back side of the carbon atom. Thus, the product obtained has the configuration opposite to that of the reactant. This is known as inversion of configuration.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




