
(a) Write the electron dot structure of calcium and sulphur.
(b) Show the formation of CaS by transfer of electrons.
Answer
525k+ views
Hint: Lewis dot structures or electron dot structures show the bonding between atoms in a molecule. Electrons are represented as dots in the Lewis structures. To draw the electron dot structure of atoms, we must know the atomic number of them. The total number of electrons represented in the Lewis dot structure will be equal to the total number of valence electrons in an atom.
Complete answer:
(a)Atomic number of calcium and sulphur are $20\,and\,16$ respectively.
Calcium is an alkaline earth metal and belongs to group $2$ & period $4$ . The electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}$ . The electron distribution in shells is given as $2,8,8,2$ . Therefore the total number of valence electrons present are $2$ . The electron dot structure of Ca is:

Sulphur is a chalcogen belonging to group $16$ & period $3$ of the periodic table. The electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$ and the electron configuration of shells is given by $2,8,6$ . Thus, total number of valence electrons are $6$ .The electron dot structure of S is:
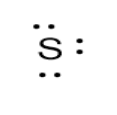
(b)Calcium has to donate its two valence electrons while sulphur has to accept a pair of electrons on its valence shell in order to attain the nearest inert gas configuration and become stable. Therefore, the transfer of electrons occur as:
$Ca\, \to \,C{a^{2 + }}\, + \,2\mathop e\limits^ -$
$ S\, + \,2\mathop e\limits^ - \to \,{S^{2 - }}$
The transfer of electrons is shown by an arrow.
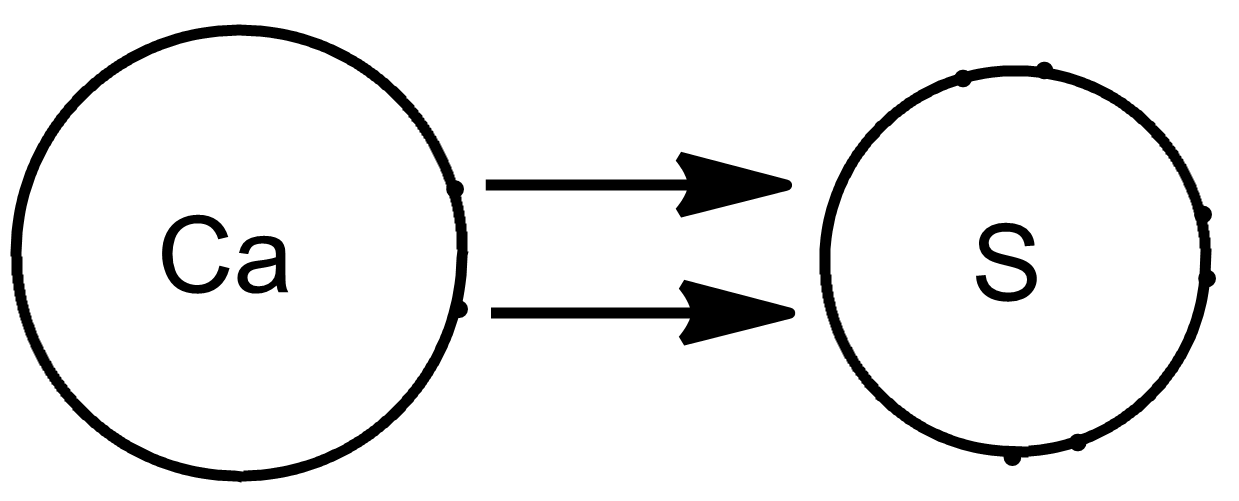
Thus, the ionic compound thus formed is Calcium sulphide is shown below.
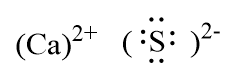
Note:
It has to be taken note that the electrons represented in a Lewis dot representation are the valence electrons of an atom i.e., the electrons present in the outermost orbital or valence shell of the atom. Non valence electrons are not depicted in this representation. Such structures can be used to represent covalently bonded and coordination compounds as well.
Complete answer:
(a)Atomic number of calcium and sulphur are $20\,and\,16$ respectively.
Calcium is an alkaline earth metal and belongs to group $2$ & period $4$ . The electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^6}4{s^2}$ . The electron distribution in shells is given as $2,8,8,2$ . Therefore the total number of valence electrons present are $2$ . The electron dot structure of Ca is:

Sulphur is a chalcogen belonging to group $16$ & period $3$ of the periodic table. The electronic configuration is $1{s^2}2{s^2}2{p^6}3{s^2}3{p^4}$ and the electron configuration of shells is given by $2,8,6$ . Thus, total number of valence electrons are $6$ .The electron dot structure of S is:
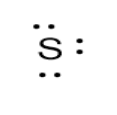
(b)Calcium has to donate its two valence electrons while sulphur has to accept a pair of electrons on its valence shell in order to attain the nearest inert gas configuration and become stable. Therefore, the transfer of electrons occur as:
$Ca\, \to \,C{a^{2 + }}\, + \,2\mathop e\limits^ -$
$ S\, + \,2\mathop e\limits^ - \to \,{S^{2 - }}$
The transfer of electrons is shown by an arrow.

Thus, the ionic compound thus formed is Calcium sulphide is shown below.
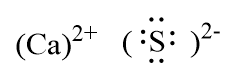
Note:
It has to be taken note that the electrons represented in a Lewis dot representation are the valence electrons of an atom i.e., the electrons present in the outermost orbital or valence shell of the atom. Non valence electrons are not depicted in this representation. Such structures can be used to represent covalently bonded and coordination compounds as well.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

Name the part of the brain responsible for the precision class 11 biology CBSE

The growth of tendril in pea plants is due to AEffect class 11 biology CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE




