
A sigma bond is formed by the overlapping of?
Answer
525k+ views
Hint: The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Complete answer:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
s – s Overlapping
One ‘s' orbital from each participating atom undergoes head-on overlapping around the internuclear axis in this form of overlapping. Before a s orbital will overlap with another, it must be half-filled.
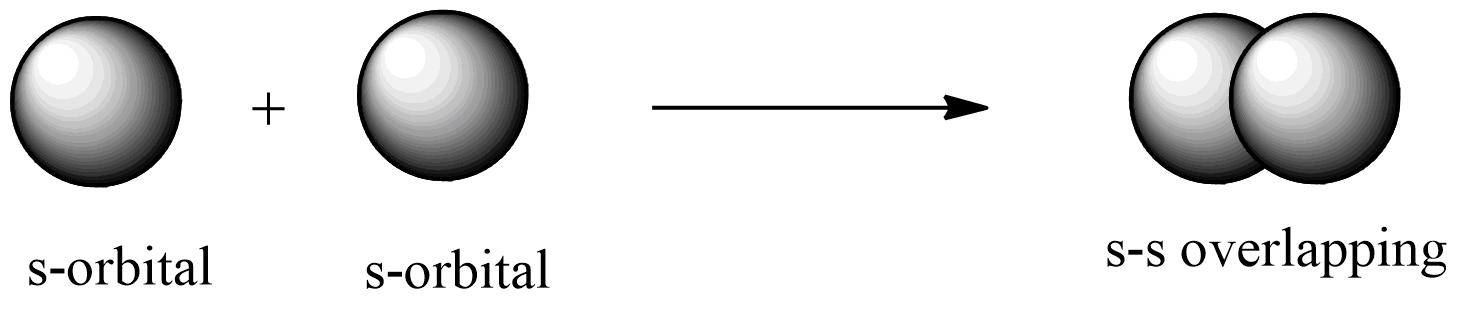
Example: Hydrogen
s – p Overlapping
Along the internuclear axis, one half-filled s orbital overlaps with one half-filled p orbital, forming a covalent bond.
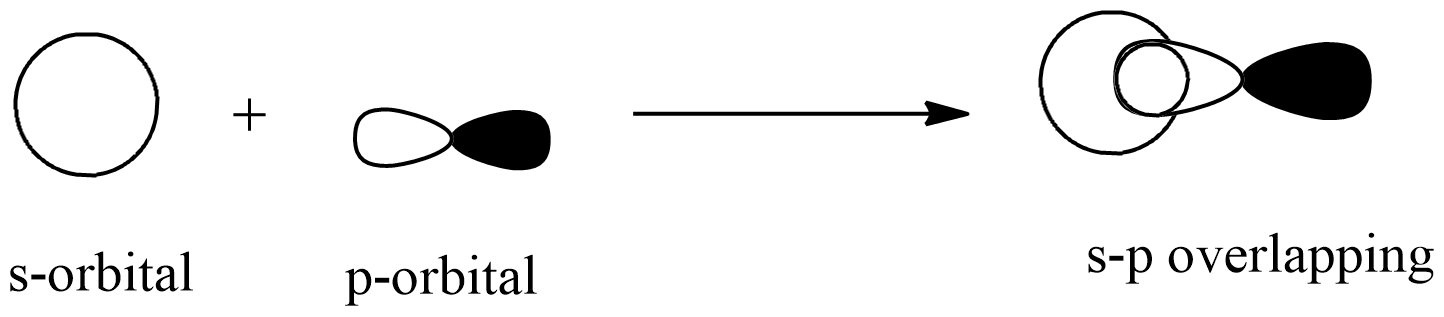
Example: Ammonia
p-p overlapping
One half-filled p orbital from each participating atom overlaps head-on along the internuclear axis in this condition.
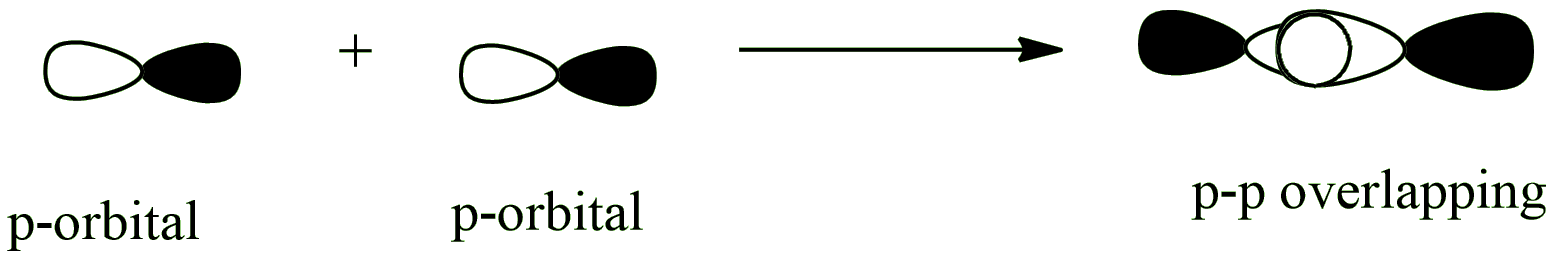
Example: Chlorine
Hence, Option C is the correct answer.
Note:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
Complete answer:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
s – s Overlapping
One ‘s' orbital from each participating atom undergoes head-on overlapping around the internuclear axis in this form of overlapping. Before a s orbital will overlap with another, it must be half-filled.
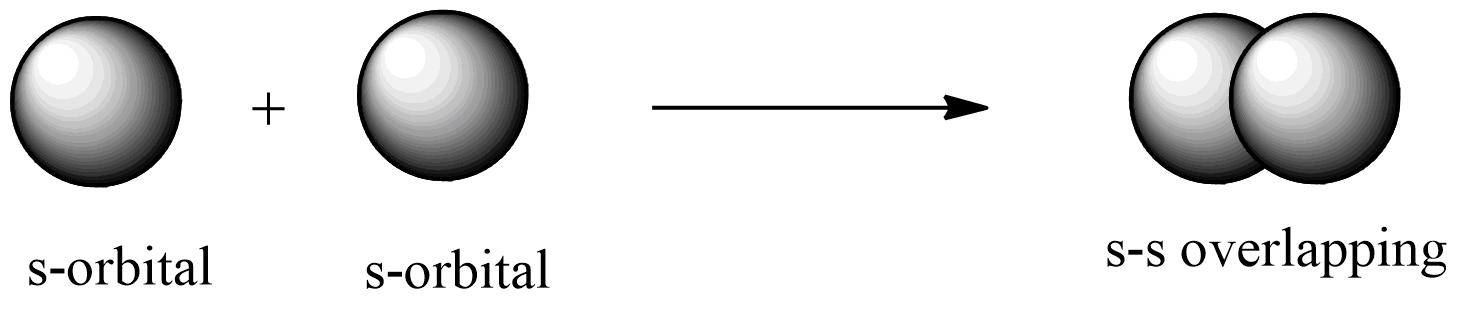
Example: Hydrogen
s – p Overlapping
Along the internuclear axis, one half-filled s orbital overlaps with one half-filled p orbital, forming a covalent bond.
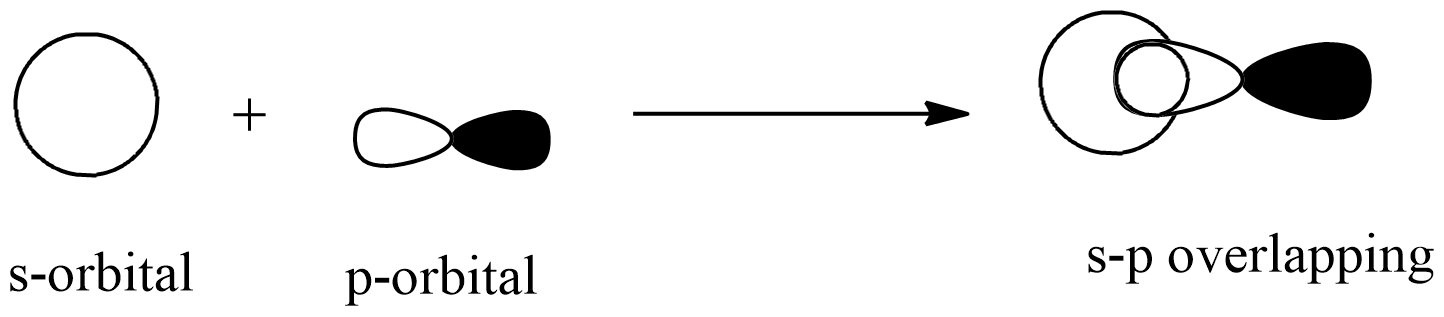
Example: Ammonia
p-p overlapping
One half-filled p orbital from each participating atom overlaps head-on along the internuclear axis in this condition.

Example: Chlorine
Hence, Option C is the correct answer.
Note:
The highest covalent chemical bond is a sigma bond. They're made up of atomic orbitals that collide head-on. For diatomic molecules, sigma bonding is best described using the terminology and resources of symmetry groups.
Both single bonds are, in general, sigma bonds. The following atomic orbital combinations may be used to make them.
Recently Updated Pages
Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 Social Science: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Chemistry: Engaging Questions & Answers for Success

Trending doubts
Draw a diagram of nephron and explain its structur class 11 biology CBSE

Explain zero factorial class 11 maths CBSE

Chemical formula of Bleaching powder is A Ca2OCl2 B class 11 chemistry CBSE

One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

A solution of a substance X is used for white washing class 11 chemistry CBSE

Differentiate between calcination and roasting class 11 chemistry CBSE




