
When a mixture of benzene and cyclohexanol is oxidized with 2-ethylanthraquinone, the product is:
A. Ethanol
B. Hydrogen peroxide
C. Anthracene
D. None of these
Answer
541.8k+ views
Hint: 2-Ethylanthraquinone is one of the organic compound which is a derivative of anthraquinone which are organic compound represented by the molecular formula${{C}_{14}}{{H}_{8}}{{O}_{2}}$. It is pale yellow in color and exists in solid form.
Complete step-by-step answer: 2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride with ethyl benzene where phthalic anhydride is represented by ${{C}_{4}}{{H}_{4}}{{(CO)}_{2}}O$and ethyl benzene is ${{C}_{6}}{{H}_{5}}Et$gives 2-Ethylanthraquinone represented by ${{C}_{6}}{{H}_{4}}{{(CO)}_{2}}{{C}_{6}}{{H}_{3}}Et$.
When 2-ethylanthraquinone dissolved in a mixture of benzene and cyclohexanol is oxidized the product formed can be explained by the following mechanism:
In step 1, 2-ethylanthraquinone is dissolved in benzene and hydrogen gas is passed through the solution in the presence of palladium represented by symbol Pd catalyst and in this process reduction takes place i.e. 2-ethylanthraquinone is reduced to 2-ethylanthraquinone.
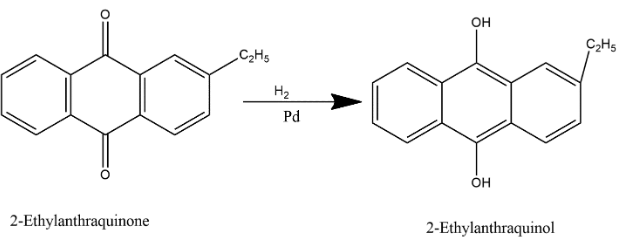
In the second step, 2-ethylanthraquinol is dissolved in mixture of benzene and cyclohexanol and when air is passed through it i.e. we supply oxygen here then oxygenation process occurs which oxidized back it to 2-ethylanthraquinone and hydrogen peroxide as shown below:
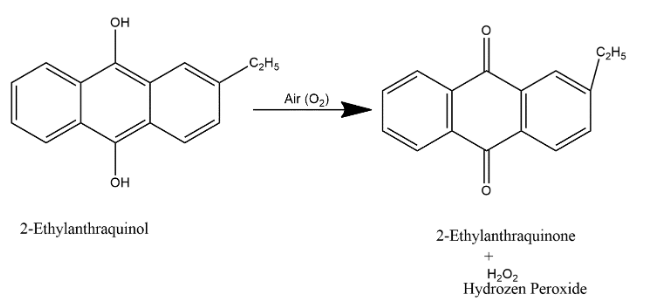
Here two products are formed 2-ethylanthraquinone and hydrogen peroxide, hence we can say that option B is the correct answer as one of the products is hydrogen peroxide.
Note: 2-ethylanthraquinone are quinone derivatives which are a type of organic compounds which can be formally derived from aromatic compounds which results in the formation of double bonded or fully conjugated cyclic diene structures.
Complete step-by-step answer: 2-Ethylanthraquinone is prepared from the reaction of phthalic anhydride with ethyl benzene where phthalic anhydride is represented by ${{C}_{4}}{{H}_{4}}{{(CO)}_{2}}O$and ethyl benzene is ${{C}_{6}}{{H}_{5}}Et$gives 2-Ethylanthraquinone represented by ${{C}_{6}}{{H}_{4}}{{(CO)}_{2}}{{C}_{6}}{{H}_{3}}Et$.
When 2-ethylanthraquinone dissolved in a mixture of benzene and cyclohexanol is oxidized the product formed can be explained by the following mechanism:
In step 1, 2-ethylanthraquinone is dissolved in benzene and hydrogen gas is passed through the solution in the presence of palladium represented by symbol Pd catalyst and in this process reduction takes place i.e. 2-ethylanthraquinone is reduced to 2-ethylanthraquinone.

In the second step, 2-ethylanthraquinol is dissolved in mixture of benzene and cyclohexanol and when air is passed through it i.e. we supply oxygen here then oxygenation process occurs which oxidized back it to 2-ethylanthraquinone and hydrogen peroxide as shown below:

Here two products are formed 2-ethylanthraquinone and hydrogen peroxide, hence we can say that option B is the correct answer as one of the products is hydrogen peroxide.
Note: 2-ethylanthraquinone are quinone derivatives which are a type of organic compounds which can be formally derived from aromatic compounds which results in the formation of double bonded or fully conjugated cyclic diene structures.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




