
A compound used as pistachio flavor in ice cream is _____.
Answer
577.8k+ views
Hint: The compound used is a type of aromatic compound or aromatic ketone. It is widely used in many ingredients in food and it imparts fragrance in cherry, strawberry almonds etc.
Complete step by step solution:
We should know that the flavoring agents are used in food to impart taste or aroma. There are chemical flavors that imitate the natural flavors. They are generally used as an additive to enhance the aroma which is generally lost during the process of food processing and also to modify the taste.
The compound that is used in pistachio flavor in ice cream is Acetophenone. It is an organic compound and the simplest aromatic ketone. Its structure is mentioned below:
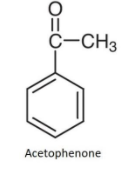
These aromatic compounds contain a ketone substituted by one alkyl group and a phenyl group. Acetophenone appears as a colorless liquid having a sweet pungent taste and odor resembling the odor of the oranges.
Acetophenone has higher density than water which means that it sinks in water. It freezes under the cool conditions. It is vapor heavier than air. It is a mild irritant to skin and eyes. Acetophenone is a sweet, almond and acacia tasting compound that can be found in a number of food items.
Note: Acetophenone can be recovered as a product of the oxidation of the ethyl benzene to the ethylbenzene hydroperoxide. Acetophenone can not be confused with benzophenone. It can be distinguished by the iodoform test since the compound benzophenone does not contain any free methyl groups hence it will give a negative iodoform test.
Complete step by step solution:
We should know that the flavoring agents are used in food to impart taste or aroma. There are chemical flavors that imitate the natural flavors. They are generally used as an additive to enhance the aroma which is generally lost during the process of food processing and also to modify the taste.
The compound that is used in pistachio flavor in ice cream is Acetophenone. It is an organic compound and the simplest aromatic ketone. Its structure is mentioned below:

These aromatic compounds contain a ketone substituted by one alkyl group and a phenyl group. Acetophenone appears as a colorless liquid having a sweet pungent taste and odor resembling the odor of the oranges.
Acetophenone has higher density than water which means that it sinks in water. It freezes under the cool conditions. It is vapor heavier than air. It is a mild irritant to skin and eyes. Acetophenone is a sweet, almond and acacia tasting compound that can be found in a number of food items.
Note: Acetophenone can be recovered as a product of the oxidation of the ethyl benzene to the ethylbenzene hydroperoxide. Acetophenone can not be confused with benzophenone. It can be distinguished by the iodoform test since the compound benzophenone does not contain any free methyl groups hence it will give a negative iodoform test.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




